Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter20: Chemistry Of Selected Transition Elements And Coordination Compounds
Section: Chapter Questions
Problem 58QRT
Related questions
Question
When an α-hydroxy amide is treated with Br2 in aqueous NaOH under Hofmann rearrangement conditions, loss of CO2 occurs and a chain-shortened aldehyde is formed. The mechanism involves the following steps:
Base abstracts an acidic amide proton, yielding amide anion 1;
The amide anion reacts with bromine in an α-substitution reaction to give N-bromoamide 2.
Abstraction of the remaining amide proton by base gives a resonance-stabilized bromoamide anion 3;
Rearrangement occurs to yield isocyanate 4;
Water adds to the isocyanate to yield carbamic acid 5;
Elimination of CO2 yields carbinolamine 6;
Following proton transfer, expulsion of ammonia yields the final product aldehyde.
Write out the mechanism
and then draw the structures of bromoamide anion 3 and amide anion 1.
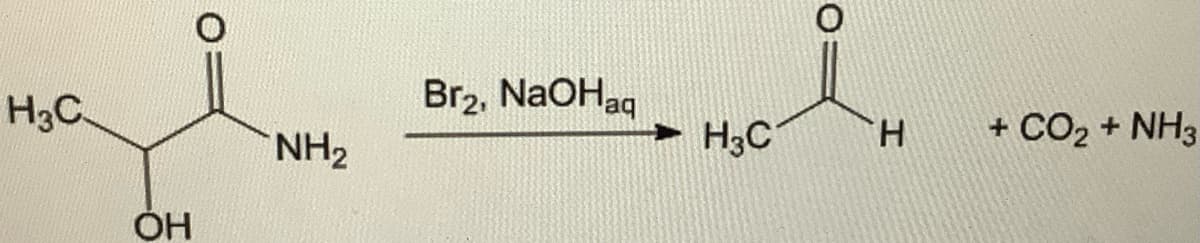
Transcribed Image Text:H3C
OH
О
NH₂
Br2, NaOHaq
H3C
Н
+ CO2 + NH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning