1. If a parcel of air at 15 °C has a specific humidity of 4.6 g/kg, what is the relative humidity of that parcel?
1. If a parcel of air at 15 °C has a specific humidity of 4.6 g/kg, what is the relative humidity of that parcel?
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question

Transcribed Image Text:Add Page
41%
5
Insert Table Chart Text Shape Media Comment
Using the equations from the last page, work out and answer the following questions:
15
1. If a parcel of air at 15 °C has a specific humidity of 4.6 g/kg, what is the relative humidity of
that parcel?
6.0
The relationship between humidity and temperature, assuming the constant humidity is that they are
inversely proportional. If temperature increases it will lead to a decrease in relative humidity.The air
will become drier, whereas when temperature decreases, the air will become wet means the relative
humidity will increase.
2. If the parcel were warmed to 25 °C with no change in water vapor content (SH), what would
be its new relative humidity?
3. Considering your answer for question 2, describe the relationship between relative humidity
and temperature, assuming constant specific humidity.
The dew point temperature of a volume of air is the temperature to which the air must be cooled to
reach a relative humidity of 100 %. Said another way, the dew point is the temperature at which a parcel
of the air becomes saturated.
4. What is the dew point temperature of an air parcel with a specific humidity of 11.2 g/kg?
1
5. A parcel of air at 20 °C has a relative humidity of 40 percent. What is its dew point
temperature?
6. Considering your responses to the questions above, do you need to know an air parcel's
temperature or its specific humidity to determine its dew point temperature?
Collaborate
Transcribed Image Text:Add Page
Temperature
°F
23
32
41
50)
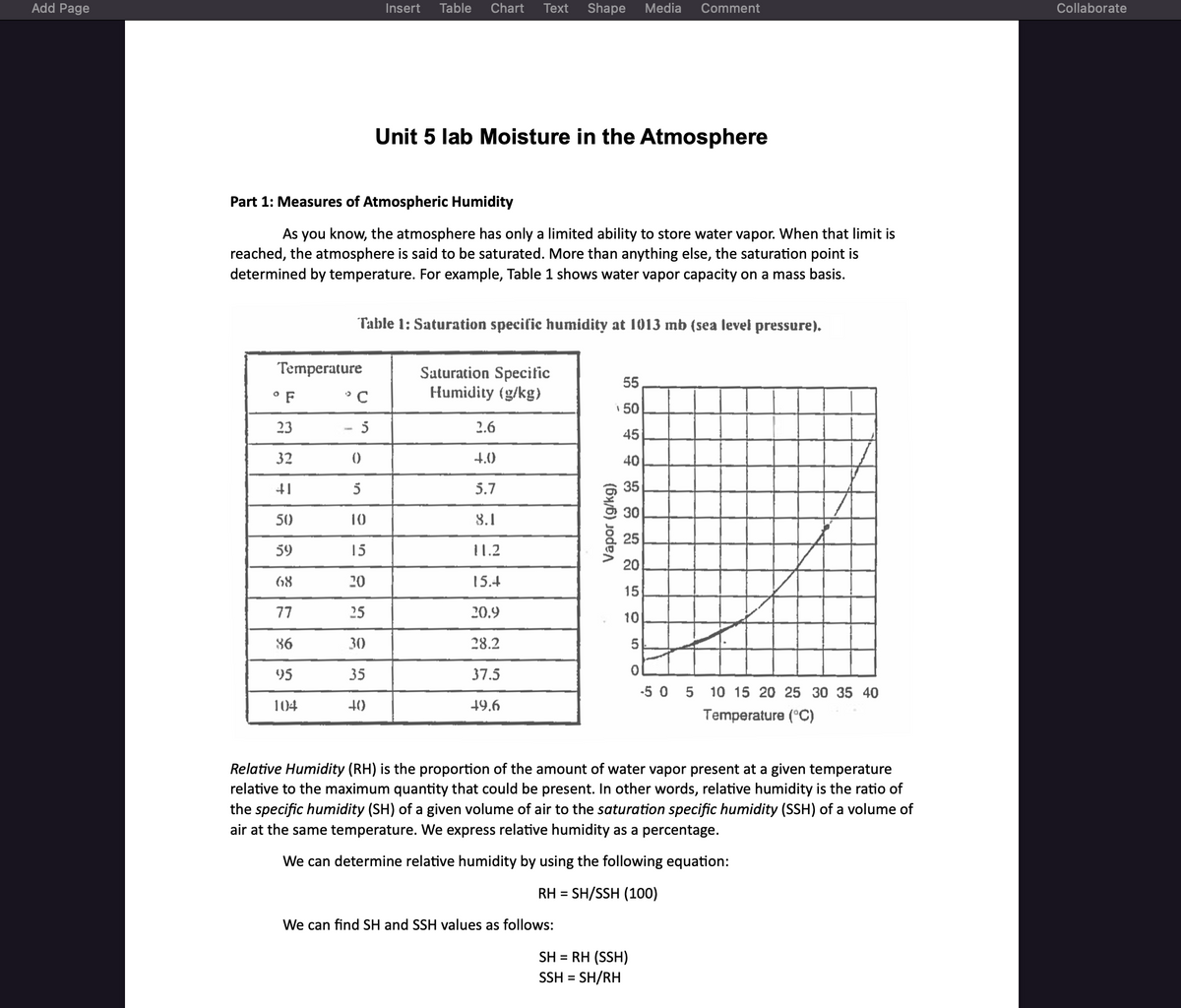
Part 1: Measures of Atmospheric Humidity
As you know, the atmosphere has only a limited ability to store water vapor. When that limit is
reached, the atmosphere is said to be saturated. More than anything else, the saturation point is
determined by temperature. For example, Table 1 shows water vapor capacity on a mass basis.
59
68
77
86
95
104
°C
5
Table 1: Saturation specific humidity at 1013 mb (sea level pressure).
()
BERGEHE
10
15
20
25
30
Insert Table
35
Chart Text Shape Media Comment
+(
Unit 5 lab Moisture in the Atmosphere
Saturation Specific
Humidity (g/kg)
2.6
4.0
5.7
8.1
11.2
15.+
20.9
28.2
37.5
49.6
55
¹50
45
40
35
30
We can find SH and SSH values as follows:
Vapor (g/kg)
25
20
15
10
5
0
SH = RH (SSH)
SSH =
= SH/RH
-5 0
Relative Humidity (RH) is the proportion of the amount of water vapor present at a given temperature
relative to the maximum quantity that could be present. In other words, relative humidity is the ratio of
the specific humidity (SH) of a given volume of air to the saturation specific humidity (SSH) of a volume of
air at the same temperature. We express relative humidity as a percentage.
We can determine relative humidity by using the following equation:
RH = SH/SSH (100)
5 10 15 20 25 30 35 40
Temperature (°C)
Collaborate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Hello can you answer the rest of the questions
Solution
Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The