Hello, sorry I think I was a bit bland with the question. In the periodic descriptions attached below, how did you come to the conclusion that the letters equalled the elements in the chart?- Which clue helped you figure that out for each element. (Provide a small explanation of how you came to the conclusion using the given properties.) Also, how does the Hydride, Chloride, and Oxide tell about the element? How is that helping us when finding the element?
Hello, sorry I think I was a bit bland with the question. In the periodic descriptions attached below, how did you come to the conclusion that the letters equalled the elements in the chart?- Which clue helped you figure that out for each element. (Provide a small explanation of how you came to the conclusion using the given properties.) Also, how does the Hydride, Chloride, and Oxide tell about the element? How is that helping us when finding the element?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 116QRT
Related questions
Question
Hello, sorry I think I was a bit bland with the question. In the periodic descriptions attached below, how did you come to the conclusion that the letters equalled the elements in the chart?- Which clue helped you figure that out for each element. (Provide a small explanation of how you came to the conclusion using the given properties.)
Also, how does the Hydride, Chloride, and Oxide tell about the element? How is that helping us when finding the element?
| Alphabet | Element | Name | Alphabet | Element | Name | Alphabet | Element | Name |
| A | C | Carbon | J | Ca | Calcium | S | Ar | Argon |
| B | F | Fluorine | K | Ne | Neon | T | Mg | Magnesium |
| C | I | Iodine | L | Be | Beryllium | U | Rb | Rubidium |
| D | Li | Lithium | M | K | Potassium | V | Ba | Barium |
| E | He | Helium | N | Ba | Barium | W | Cl | Chlorine |
| F | Fr | Francium | P | Kr | Krypton | X | Sn | Tin |
| G | Xe | Xenon | Q | Ge | Germanium | Y | Pb | Lead |
| I | Si | Silicon | R | Br | Bromine | Z | Na | Sodium |
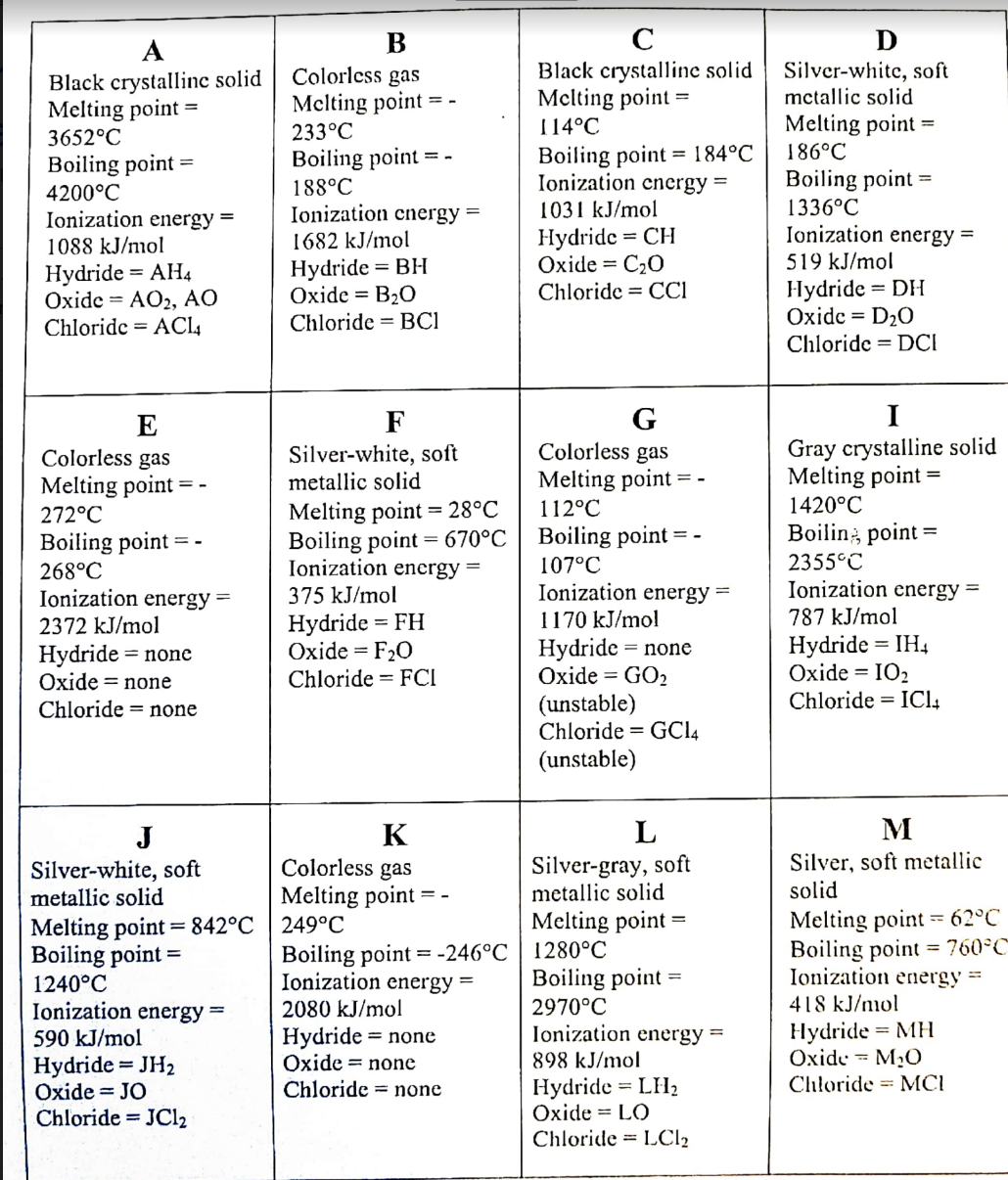
Transcribed Image Text:D
Silver-white, soft
mctallic solid
A
B
Black crystallinc solid
Melting point
Colorless gas
Melting point
233°C
Black crystalline solid
Melting point =
Melting point =
186°C
114°C
3652°C
Boiling point = 184°C
Ionization cnergy =
Boiling point
4200°C
Ionization energy =
1088 kJ/mol
Boiling point
188°C
Ionization cnergy =
1682 kJ/mol
Boiling point =
1031 kJ/mol
1336°C
Hydride = AH4
Oxide = AO2, AO
Chloride = ACI4
Hydride = BH
Oxide = B20
Chloride = BCI
Hydride = CH
Oxide = C20
Chloride = CCI
Ionization energy =
519 kJ/mol
Hydride = DH
Oxide = D20
Chloride = DCI
I
G
Colorless gas
Melting point =
112°C
E
F
Silver-white, soft
metallic solid
Gray crystalline solid
Melting point
Colorless gas
Melting point
1420°C
Melting point = 28°C
Boiling point = 670°C
Ionization energy =
272°C
Boiling point
Boiling point
Boilin; point =
2355°C
268°C
Ionization energy -
2372 kJ/mol
107°C
Ionization energy
1170 kJ/mol
375 kJ/mol
Ionization energy =
787 kJ/mol
Hydride = FH
Oxide = F20
Chloride = FCI
Hydride = none
Oxide = GO2
(unstable)
Chloride = GCI4
(unstable)
Нydride %3DIH4
Oxide = IO2
Chloride = ICl,
Hydride = none
Oxide = none
Chloride = none
J
K
M
Silver-gray, soft
metallic solid
Silver, soft metallic
solid
Colorless gas
Silver-white, soft
metallic solid
Melting point
= -
Melting point
Boiling point = -246°C | 1280°C
Boiling point
Melting point = 842°C | 249°C
Boiling point
1240°C
Ionization energy =
590 kJ/mol
Melting point -- 62°C
Boiling point = 760°C
Ionization energy =
418 kJ/mol
Hydride = MH
Oxide = M2O
Chloride = MCI
%3D
%3D
Ionization energy =
2970°C
Ionization energy =
2080 kJ/mol
Hydride = none
Oxide = none
Chloride = none
898 kJ/mol
Hydride = JH2
Oxide = JO
Chloride = JC12
Hydride = LH2
Oxide = LO
Chloride = LC12
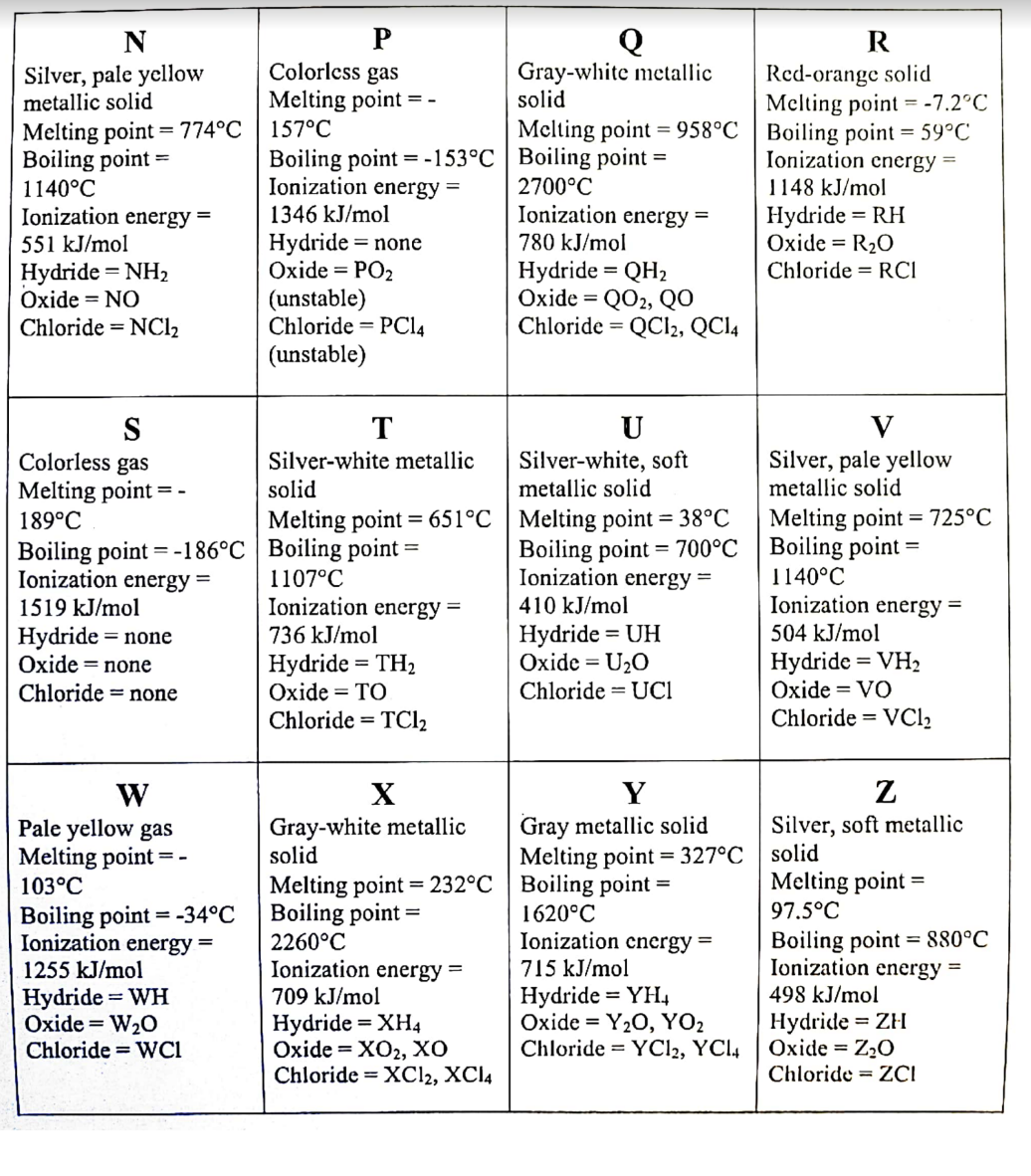
Transcribed Image Text:N
R
Colorless gas
Melting point
Gray-white metallic
solid
Red-orange solid
Melting point =-7.2°C
Melting point = 958°C | Boiling point = 59°C
Ionization cnergy =
Silver, pale yellow
metallic solid
Melting point = 774°C | 157°C
Boiling point
1140°C
Ionization energy =
Boiling point = -153°C | Boiling point
2700°C
Ionization energy =
Ionization energy =
1148 kJ/mol
Hydride = RH
Oxide = R2O
1346 kJ/mol
Hydride = none
Oxide = PO2
551 kJ/mol
780 kJ/mol
%3D
Hydride = NH2
Oxide = NO
Hydride = QH2
Охide %3D QO, QO
Chloride = QCI2, QC14
Chloride = RCI
(unstable)
Chloride = PC14
(unstable)
Chloride = NC12
S
Colorless gas
Melting point
189°C
U
Silver-white, soft
metallic solid
V
Silver, pale yellow
metallic solid
T
Silver-white metallic
solid
Melting point = 651°C | Melting point = 38°C
Melting point = 725°C
Boiling point = -186°C | Boiling point =
Ionization energy =
Boiling point = 700°C | Boiling point =
Ionization energy =
%3D
1140°C
1107°C
Ionization energy =
736 kJ/mol
1519 kJ/mol
410 kJ/mol
Ionization energy =
Hydride = UH
Oxide = U20
Chloride = UCI
504 kJ/mol
Hydride = none
Oxide = none
Hydride = TH2
Oxide = TO
Chloride = TCI2
Hydride = VH2
Oxide = VO
Chloride = none
Chloride = VC12
X
Y
W
Pale yellow gas
Melting point =-
103°C
Gray-white metallic
solid
Gray metallic solid
Melting point = 327°C
Melting point = 232°C | Boiling point =
Silver, soft metallic
solid
Melting point =
97.5°C
Boiling point = -34°C
Ionization energy =
1255 kJ/mol
Boiling point =
1620°C
Ionization cnergy =
%3D
Boiling point = 880°C
Ionization energy =
2260°C
%3D
Ionization energy =
715 kJ/mol
Hydride = YH,
Oxide = Y20, YO2
Chloride = YC12, YCI4
498 kJ/mol
Hydride = WH
Oxide = W20
Chloride = WCi
709 kJ/mol
Hydride = XH4
Оxide%3D XOz, хо
Chloride = XC12, XCI4
Hydride = ZH
Oxide = Z20
Chloride = ZCI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning