Here I have numbèred the atoms in the imidazole ring for you. Notice that the N at position #4 at the top of the ring does not have a lone pair outside the ring. To satisfy Huckel's Rule, that lone pair has to be inside the ring, meaning it would not be available for hydrogen bonding. histidine NH NH2 serine но- но. 3 INH2 active site 2 H- regenerated R. If the imidazole ring were rotated 180 degrecs, so that #4 is down and # 2 is up, then the hydrogen bond with the H on the serine hydroxyl group would not happen. But a hydrogen bond between the O of serinc and the H on N # 4 could happen. There must be something else keeping the imidazole ring oriented as shown. The P domain is arranged so that there is an aspartate side chain just above the imidazole ring. What is the charge of the aspartate side chain at physiologic pH? (Please look up the pKa on the internet) +1 O neutral
Here I have numbèred the atoms in the imidazole ring for you. Notice that the N at position #4 at the top of the ring does not have a lone pair outside the ring. To satisfy Huckel's Rule, that lone pair has to be inside the ring, meaning it would not be available for hydrogen bonding. histidine NH NH2 serine но- но. 3 INH2 active site 2 H- regenerated R. If the imidazole ring were rotated 180 degrecs, so that #4 is down and # 2 is up, then the hydrogen bond with the H on the serine hydroxyl group would not happen. But a hydrogen bond between the O of serinc and the H on N # 4 could happen. There must be something else keeping the imidazole ring oriented as shown. The P domain is arranged so that there is an aspartate side chain just above the imidazole ring. What is the charge of the aspartate side chain at physiologic pH? (Please look up the pKa on the internet) +1 O neutral
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter23: Amines
Section: Chapter Questions
Problem 23.21P
Related questions
Question
3
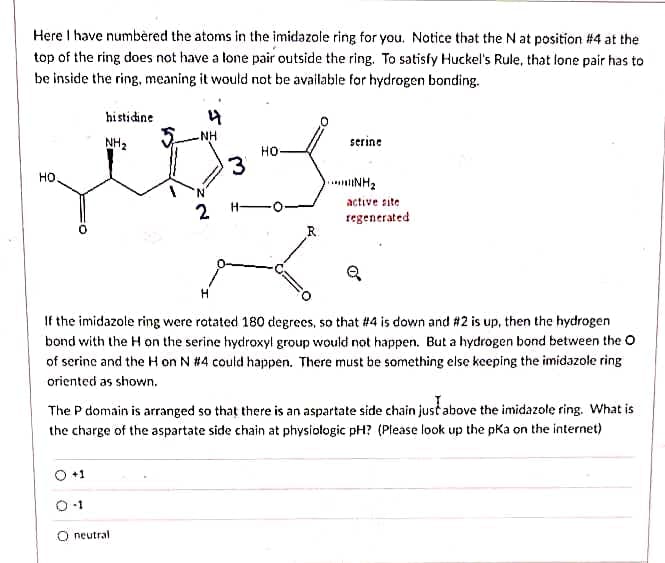
Transcribed Image Text:Here I have numbèred the atoms in the imidazole ring for you. Notice that the N at position #4 at the
top of the ring does not have a lone pair outside the ring. To satisfy Huckel's Rule, that lone pair has to
be inside the ring, meaning it would not be available for hydrogen bonding.
histidine
NH
NH2
serine
но-
но.
NH2
active site
2 H-
regenerated
If the imidazole ring were rotated 180 degrees, so that #4 is down and #2 is up, then the hydrogen
bond with the H on the serine hydroxyl group would not happen. But a hydrogen bond between the O
of serine and the H on N #4 could happen. There must be something else keeping the imidazole ring
oriented as shown.
The P domain is arranged so that there is an aspartate side chain just above the imidazole ring. What is
the charge of the aspartate side chain at physiołogic pH? (Please look up the pKa on the internet)
O+1
O neutral
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning