History Bookmarks People Tab Window Help Edit View Question 3 - Chapter 5 - Conn x |- Adams, Amaya K - OutloX B Chapter 5- CHEM-1110-941- zto.mheducation.com/ext/map/index.html?_con3con&external_browser%3D0&launchUrl=https%253A%252F%252Flms.mheducation Saved r 5 6 3 attempts left Check my work Enter your answer in the provided box. Dry ice is solid carbon dioxide. A 1.43-g sample of dry ice is placed in an evacuated 3.93–L vessel at 22.0°C. Calculate the pressure inside the vessel after all the dry ice has been converted to CO2 gas. So Guide atm ces
History Bookmarks People Tab Window Help Edit View Question 3 - Chapter 5 - Conn x |- Adams, Amaya K - OutloX B Chapter 5- CHEM-1110-941- zto.mheducation.com/ext/map/index.html?_con3con&external_browser%3D0&launchUrl=https%253A%252F%252Flms.mheducation Saved r 5 6 3 attempts left Check my work Enter your answer in the provided box. Dry ice is solid carbon dioxide. A 1.43-g sample of dry ice is placed in an evacuated 3.93–L vessel at 22.0°C. Calculate the pressure inside the vessel after all the dry ice has been converted to CO2 gas. So Guide atm ces
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter3: Chemical Reactions
Section3.9: Classifying Reactions In Aqueous Solution
Problem 2.2ACP
Related questions
Question
Transcribed Image Text:Bookmarks
People
Tab
Window
Help
Edit
View
History
O Question 3 - Chapter 5 - Conn x
+
ail - Adams, Amaya K - Outlo x
B Chapter 5 - CHEM-1110-941
ezto.mheducation.com/ext/map/index.html?_con=con&external_browser=0&launchUrl=https%253A%252F%252Flms.mheducation
Saved
er 5 A
3 attempts left
Check my work
Enter your answer in the provided box.
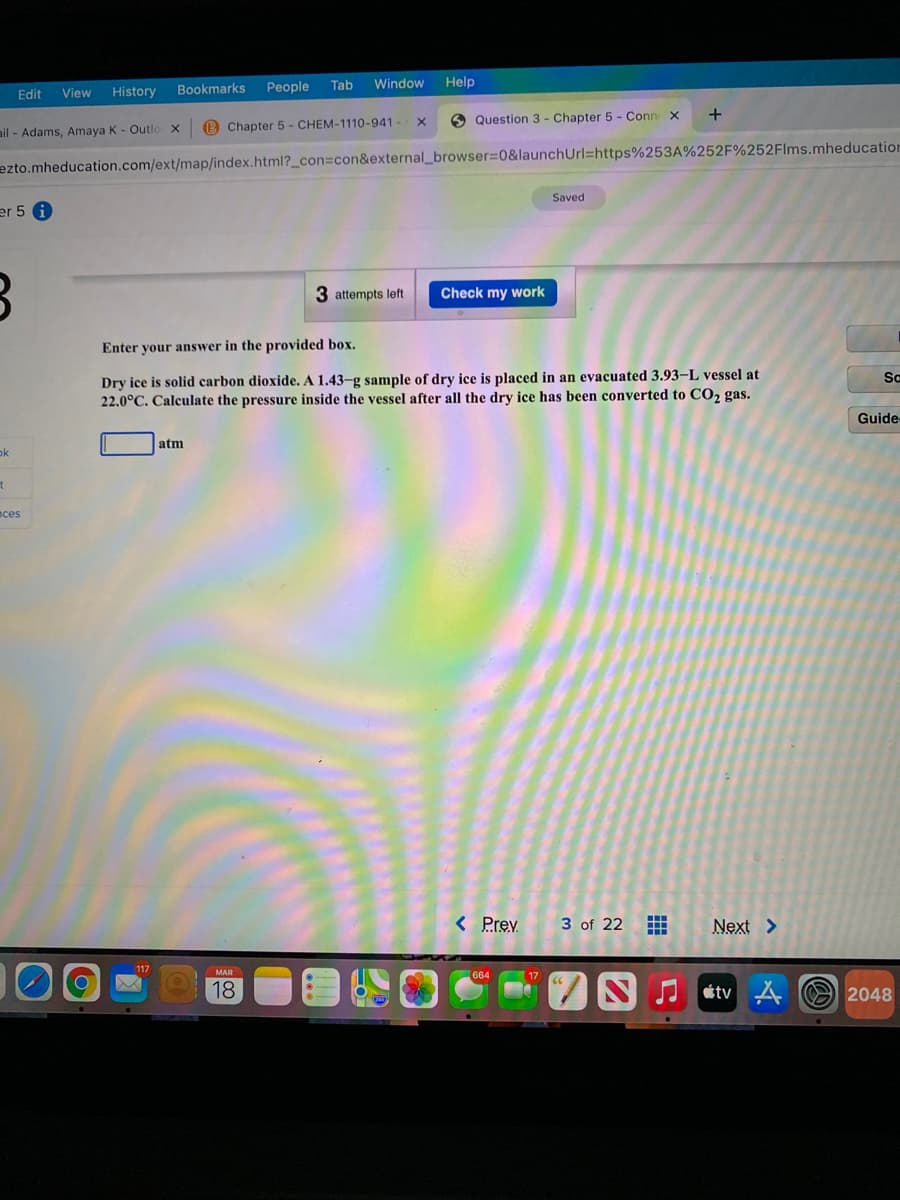
Dry ice is solid carbon dioxide. A 1.43-g sample of dry ice is placed in an evacuated 3.93–L vessel at
22.0°C. Calculate the pressure inside the vessel after all the dry ice has been converted to CO2 gas.
Sc
Guide
atm
ok
nces
< Prev
3 of 22
Next >
МAR
664
18
I tv A O 2048
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning