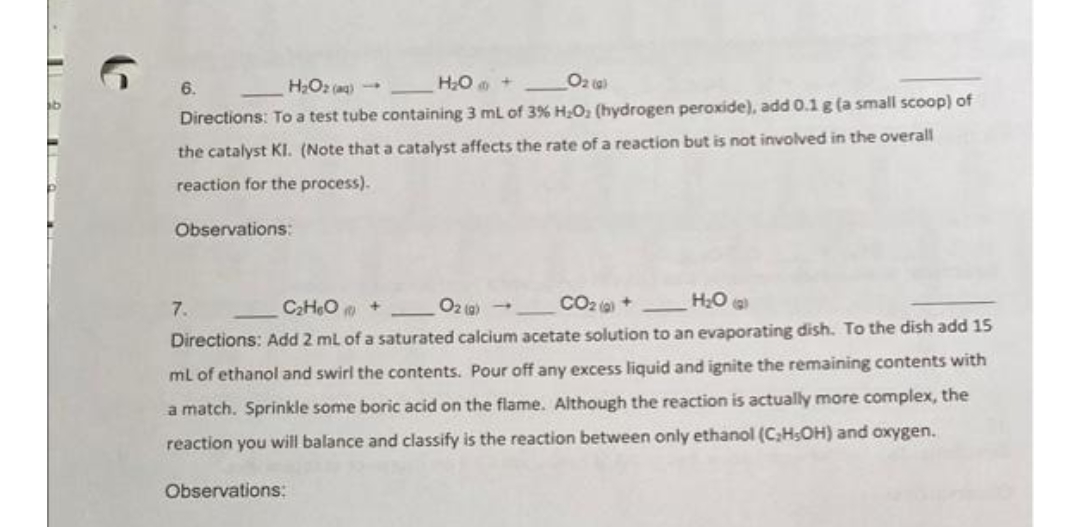
HO + Oz ta 6. Directions: To a test tube containing 3 mL of 3% H.O; (hydrogen peroxide), add 0.1 g (a small scoop) of the catalyst KI. (Note that a catalyst affects the rate of a reaction but is not involved in the overall reaction for the process). Observations: 7. C2HeO + O2 (g) - CO2 0 + H:O Directions: Add 2 ml of a saturated calcium acetate solution to an evaporating dish. To the dish add 15 ml of ethanol and swirl the contents. Pour off any excess liquid and ignite the remaining contents with a match. Sprinkle some boric acid on the flame. Although the reaction is actually more complex, the reaction you will balance and classify is the reaction between only ethanol (CHSOH) and oxygen. Observations:
HO + Oz ta 6. Directions: To a test tube containing 3 mL of 3% H.O; (hydrogen peroxide), add 0.1 g (a small scoop) of the catalyst KI. (Note that a catalyst affects the rate of a reaction but is not involved in the overall reaction for the process). Observations: 7. C2HeO + O2 (g) - CO2 0 + H:O Directions: Add 2 ml of a saturated calcium acetate solution to an evaporating dish. To the dish add 15 ml of ethanol and swirl the contents. Pour off any excess liquid and ignite the remaining contents with a match. Sprinkle some boric acid on the flame. Although the reaction is actually more complex, the reaction you will balance and classify is the reaction between only ethanol (CHSOH) and oxygen. Observations:
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter13: Fundamental Equilibrium Concepts
Section: Chapter Questions
Problem 96E: The binding of oxygen by hemoglobin (Hb), giving oxy-hemoglobin (HbO2), is partially regulated by...
Related questions
Question
Transcribed Image Text:HO +
Oz ta)
6.
Directions: To a test tube containing 3 ml of 3% H.O, (hydrogen peroxide), add 0.1 g (a small scoop) of
the catalyst KI. (Note that a catalyst affects the rate of a reaction but is not involved in the overall
reaction for the process).
Observations:
7.
C2HeO +
O2 (9) -
CO2 (a) +
Directions: Add 2 ml of a saturated calcium acetate solution to an evaporating dish. To the dish add 15
ml of ethanol and swirl the contents. Pour off any excess liquid and ignite the remaining contents with
a match. Sprinkle some boric acid on the flame. Although the reaction is actually more complex, the
reaction you will balance and classify is the reaction between only ethanol (C,HSOH) and oxygen.
Observations:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning