Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter22: Synthesis Workshop 2
Section: Chapter Questions
Problem 5CTQ
Related questions
Question
chem hw
![Alkyl Halides (X = CI, Br or I): Assume AICI, is present if needed
2 of 2
G
un acid catalyst [H+], or pyridine, is present if needed.
он
OH
OH
CHJOH
AA
BB
DD
EE
FF
GG
HH
Ketones, Aldehydes and Epoxides: Assume "then H,0" is included if a protonation step is needed
K.
P
R
V
Acid Chlorides: Assume AICI, or pyridine is present if needed
YY
zZ
Other Reagents:
11 PCC in CH2C2
21 Br2, FeBr3
22 Mg. Et,0
23 Cl2, AICI3
24 SOCI2, pyridine
25 HNO3, H2SO4
26 fuming H2SO4
1 H3O* (dilute H2SO4) or H3O*, heat
2 conc. H2SO4, heat
3 NaOEt
12 NazCr207, H2S04, H2O
13 BH3•THF or 9-BBN, then H2O2, NaOH
14 Hg(OAc)2, H2O, then NaBH4
15 O3, then Zn, HCI or DMS
16 MCPBA or CH;CO3H
17 Br2, light or NBS, heat
4 t-BUOK
5 H2, Pt
6 H2, Lindlar's catalyst
7 Na, NH3
8 LAH or xs LAH, then H20
9 NABH4, CH3OH
10 NABH,CN, pH 5
27 Fe, HCI; then NaOH
28 Zn(Hg). НCI
29 KCN, or KCN + HCN
30 CO2, then H30*
18 HBr
19 HBr, ROOR
20 PB13
31 (H*]. HOʻ
32 NH3 (1 or 2 equiv.)
33 CH,NH2 (1 or 2 equiv)
34 (CH3)½NH (1 or 2 equiv)
35 EINH2 (1 or 2 equiv)
36 PHCH,NH2 (1 or 2 equiv).
37 LDA, -78 °C
(-H20)
Grignard, Wittig and Gilman Reagents:
Assume "then H,0" is included if a protonation step is needed
MgBr
MeMgBr
EtMgBr
PhMgBr
G1
G2
G3
G4
CuLi
Me,Culi Et,Culi (PHCH2),CULI
38 NaH, 25 °C
39 LIAI(OR);H, then H20
40 DIBAH, then H2O
41 Br2. [H3O*]
42 Br2, NaOH
43 Pyridine
G5
G6
G7
G8
MePh,P=CH2 PhyP=CHCH3 PhyP=CHCO,Et PhyP=CHPH
W1
w2
W3
W4](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fbc96294a-6867-4c53-8945-7b1c26d22cf9%2F2c5f0633-970d-4d43-9245-741e4a40b6b3%2F8tvr7b_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Alkyl Halides (X = CI, Br or I): Assume AICI, is present if needed
2 of 2
G
un acid catalyst [H+], or pyridine, is present if needed.
он
OH
OH
CHJOH
AA
BB
DD
EE
FF
GG
HH
Ketones, Aldehydes and Epoxides: Assume "then H,0" is included if a protonation step is needed
K.
P
R
V
Acid Chlorides: Assume AICI, or pyridine is present if needed
YY
zZ
Other Reagents:
11 PCC in CH2C2
21 Br2, FeBr3
22 Mg. Et,0
23 Cl2, AICI3
24 SOCI2, pyridine
25 HNO3, H2SO4
26 fuming H2SO4
1 H3O* (dilute H2SO4) or H3O*, heat
2 conc. H2SO4, heat
3 NaOEt
12 NazCr207, H2S04, H2O
13 BH3•THF or 9-BBN, then H2O2, NaOH
14 Hg(OAc)2, H2O, then NaBH4
15 O3, then Zn, HCI or DMS
16 MCPBA or CH;CO3H
17 Br2, light or NBS, heat
4 t-BUOK
5 H2, Pt
6 H2, Lindlar's catalyst
7 Na, NH3
8 LAH or xs LAH, then H20
9 NABH4, CH3OH
10 NABH,CN, pH 5
27 Fe, HCI; then NaOH
28 Zn(Hg). НCI
29 KCN, or KCN + HCN
30 CO2, then H30*
18 HBr
19 HBr, ROOR
20 PB13
31 (H*]. HOʻ
32 NH3 (1 or 2 equiv.)
33 CH,NH2 (1 or 2 equiv)
34 (CH3)½NH (1 or 2 equiv)
35 EINH2 (1 or 2 equiv)
36 PHCH,NH2 (1 or 2 equiv).
37 LDA, -78 °C
(-H20)
Grignard, Wittig and Gilman Reagents:
Assume "then H,0" is included if a protonation step is needed
MgBr
MeMgBr
EtMgBr
PhMgBr
G1
G2
G3
G4
CuLi
Me,Culi Et,Culi (PHCH2),CULI
38 NaH, 25 °C
39 LIAI(OR);H, then H20
40 DIBAH, then H2O
41 Br2. [H3O*]
42 Br2, NaOH
43 Pyridine
G5
G6
G7
G8
MePh,P=CH2 PhyP=CHCH3 PhyP=CHCO,Et PhyP=CHPH
W1
w2
W3
W4
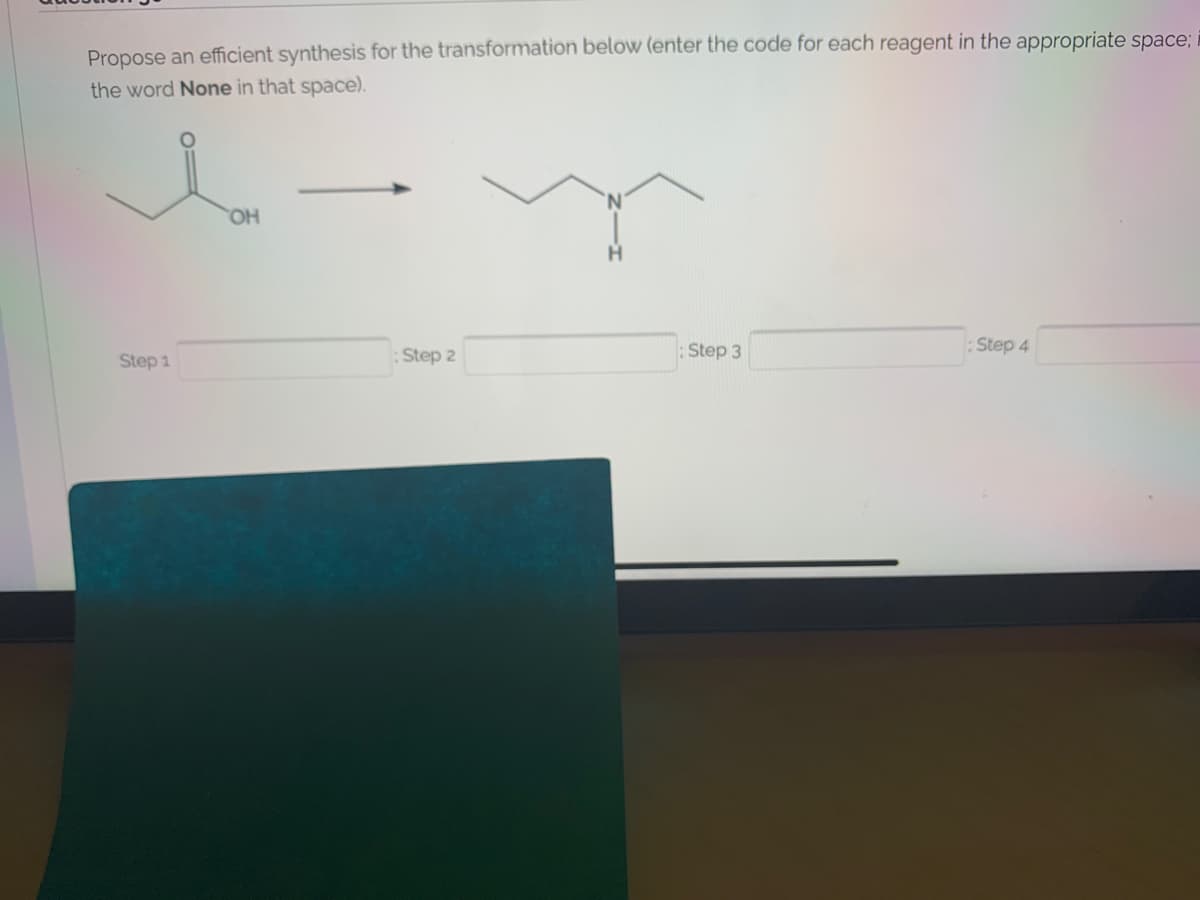
Transcribed Image Text:Propose an efficient synthesis for the transformation below (enter the code for each reagent in the appropriate space;
the word None in that space).
HO.
:Step 3
:Step 4
Step 1
:Step 2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning