Chapter10: Reconstitution Of Powdered Drugs
Section: Chapter Questions
Problem 7.2P
Related questions
Question
100%
How can I simplify the procedure section to be prepared for lab without having to refer to the lab manual?
Transcribed Image Text:dee os to
dasem
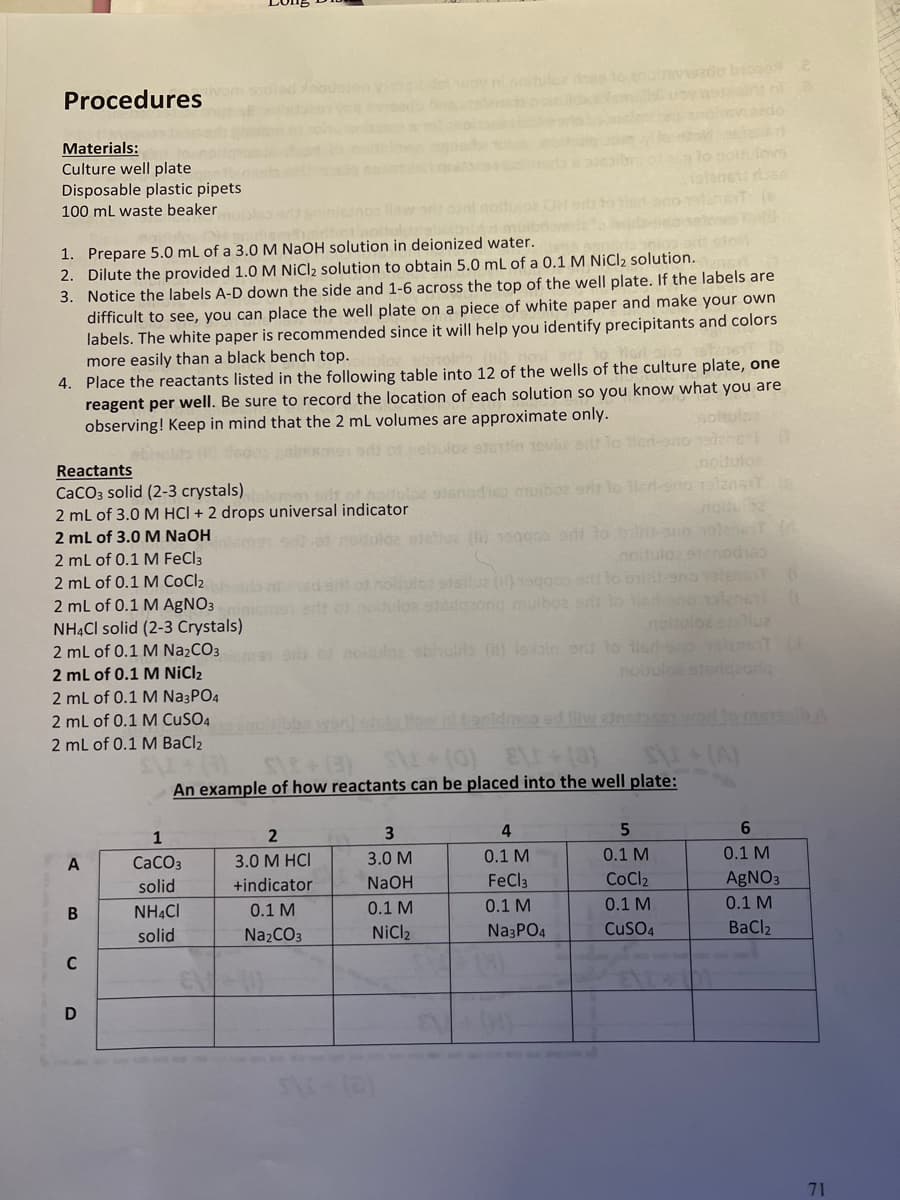
Procedures
edo
leseo
Materials:
Culture well plate
Hove
Disposable plastic pipets
100 mL waste beaker
1. Prepare 5.0 mL of a 3.0 M NaOH solution in deionized water.
2. Dilute the provided 1.0 M NiCl2 solution to obtain 5.0 mL of a 0.1 M NİCI2 solution.
3. Notice the labels A-D down the side and 1-6 across the top of the well plate. If the labels are
difficult to see, you can place the well plate on a piece of white paper and make your own
labels. The white paper is recommended since it will help you identify precipitants and colors
more easily than a black bench top.
4. Place the reactants listed in the following table into 12 of the wells of the culture plate, one
reagent per well. Be sure to record the location of each solution so you know what you are
observing! Keep in mind that the 2 mL volumes are approximate only.
noltulo
Reactants
CaCO3 solid (2-3 crystals)
oulbe otsnadeo muiboe eri lo led-snoelanat
2 mL of 3.0 M HCI + 2 drops universal indicator
2 ml of 3.0 M NaOH
2 ml of 0.1 M FeCl3
noitulo ienodico
nolulos stsiluR )ggos s to bleno en
o noituloa sddgaorig muiboa s to iedno lene0
noltuloe suz
2 ml of 0.1 M NażCO3 s nouloe sbholds () labin ord to lied-ono lensiT
noluloz sterngzorlg
2 mL of 0.1 M COCI2
2 ml of 0.1 M AgNO3
NHẠCI solid (2-3 Crystals)
2 ml of 0.1 M NİCI2
2 ml of 0.1 M Na3PO4
2 ml of 0.1 M CUSO4
aldmo od liiv einnt wodo.melbA
2 ml of 0.1 M BaCl2
S+ S (0)
An example of how reactants can be placed into the well plate:
4
6.
1
CaCO3
3.0 М HСI
3.0 M
0.1 M
0.1 M
0.1 M
A
solid
+indicator
N2OH
FeCl3
CoCl2
AgNO3
В
NHẠCI
0.1 M
0.1 M
0.1 M
0.1 M
0.1 M
solid
NazCO3
NiCl2
Na3PO4
CusO4
BaCl2
71
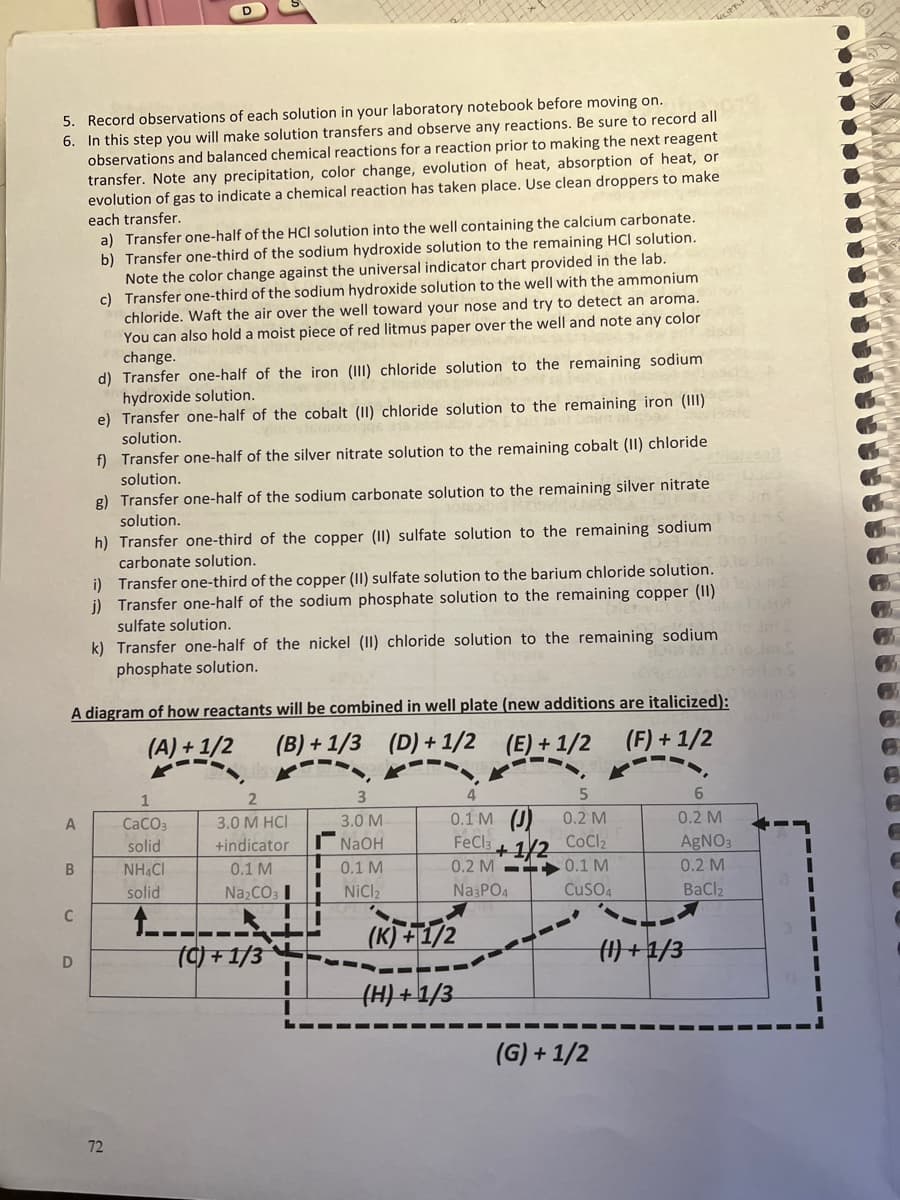
Transcribed Image Text:5. Record observations of each solution in your laboratory notebook before moving on.
6. In this step you will make solution transfers and observe any reactions. Be sure to record all
observations and balanced chemical reactions for a reaction prior to making the next reagent
transfer. Note any precipitation, color change, evolution of heat, absorption of heat, or
evolution of gas to indicate a chemical reaction has taken place. Use clean droppers to make
each transfer.
a) Transfer one-half of the HCl solution into the well containing the calcium carbonate.
b) Transfer one-third of the sodium hydroxide solution to the remaining HCI solution.
Note the color change against the universal indicator chart provided in the lab.
c) Transfer one-third of the sodium hydroxide solution to the well with the ammonium
chloride. Waft the air over the well toward your nose and try to detect an aroma.
You can also hold a moist piece of red litmus paper over the well and note any color
change.
d) Transfer one-half of the iron (III) chloride solution to the remaining sodium
hydroxide solution.
e) Transfer one-half of the cobalt (II) chloride solution to the remaining iron (III)
solution.
f) Transfer one-half of the silver nitrate solution to the remaining cobalt (II) chloride
solution.
g) Transfer one-half of the sodium carbonate solution to the remaining silver nitrate
solution.
h) Transfer one-third of the copper (II) sulfate solution to the remaining sodium
carbonate solution.
i) Transfer one-third of the copper (II) sulfate solution to the barium chloride solution.
i) Transfer one-half of the sodium phosphate solution to the remaining copper (II)
sulfate solution.
k) Transfer one-half of the nickel (II) chloride solution to the remaining sodium
phosphate solution.
A diagram of how reactants will be combined in well plate (new additions are italicized):
(A) +1/2
(B) + 1/3 (D) + 1/2
(E) + 1/2 (F) + 1/2
1
0.1 M (J)
FeCla
A
CaCO3
3.0 M HCI
3.0 M
0.2 M
0.2 M
+1/2 CoCl2
0.2 M -1 0.1 M
solid
+indicator
NaOH
AGNO3
NH CI
0.1 M
0.1 M
0.2 M
solid
Na2CO3 I
NICI2
Na PO4
Cuso4
BaCl2
t----
(C) + 1/3
(K) +T/2
D
(H) +1/3
(G) + 1/2
72
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

