Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:Elaine N. Marieb, Katja N. Hoehn
Chapter1: The Human Body: An Orientation
Section: Chapter Questions
Problem 1RQ: The correct sequence of levels forming the structural hierarchy is A. (a) organ, organ system,...
Related questions
Question
How can we tell if the enzyme has been denatured by a particular temperature treatment.
Transcribed Image Text:oft Word - Lab 5 Enzymat X
Download Firefox Browser- Fas X
/Downloads/Lab%205%20Enzymatic%20Activity%20(9).pdf
Getting Started
TV shows
ted From Fire
Swift - Login
Vision Loss Resourc
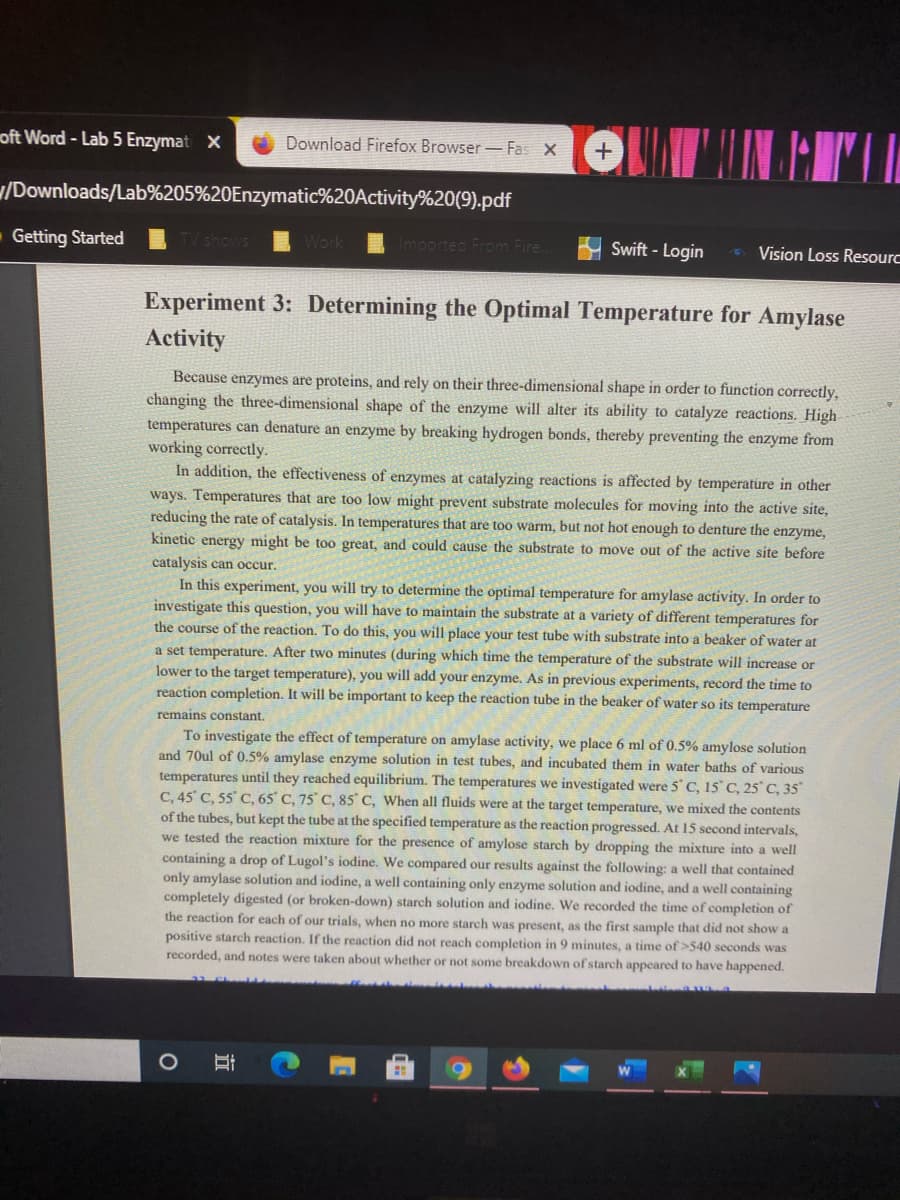
Experiment 3: Determining the Optimal Temperature for Amylase
Activity
Because enzymes are proteins, and rely on their three-dimensional shape in order to function correctly,
changing the three-dimensional shape of the enzyme will alter its ability to catalyze reactions. High
temperatures can denature an enzyme by breaking hydrogen bonds, thereby preventing the enzyme from
working correctly.
In addition, the effectiveness of enzymes at catalyzing reactions is affected by temperature in other
ways. Temperatures that are too low might prevent substrate molecules for moving into the active site,
reducing the rate of catalysis. In temperatures that are too warm, but not hot enough to denture the enzyme,
kinetic energy might be too great, and could cause the substrate to move out of the active site before
catalysis can occur.
In this experiment, you will try to determine the optimal temperature for amylase activity. In order to
investigate this question, you will have to maintain the substrate at a variety of different temperatures for
the course of the reaction. To do this, you will place your test tube with substrate into a beaker of water at
a set temperature. After two minutes (during which time the temperature of the substrate will increase or
lower to the target temperature), you will add your enzyme. As in previous experiments, record the time to
reaction completion. It will be important to keep the reaction tube in the beaker of water so its temperature
remains constant.
To investigate the effect of temperature on amylase activity, we place 6 ml of 0.5% amylose solution
and 70ul of 0.5% amylase enzyme solution in test tubes, and incubated them in water baths of various
temperatures until they reached equilibrium. The temperatures we investigated were 5 C, 15 C, 25° C, 35°
C, 45 C, 55 C, 65' C, 75° C, 85 C, When all fluids were at the target temperature, we mixed the contents
of the tubes, but kept the tube at the specified temperature as the reaction progressed. At 15 second intervals,
we tested the reaction mixture for the presence of amylose starch by dropping the mixture into a well
containing a drop of Lugol's iodine. We compared our results against the following: a well that contained
only amylase solution and iodine, a well containing only enzyme solution and iodine, and a well containing
completely digested (or broken-down) starch solution and iodine. We recorded the time of completion of
the reaction for each of our trials, when no more starch was present, as the first sample that did not show a
positive starch reaction. If the reaction did not reach completion in 9 minutes, a time of >540 seconds was
recorded, and notes were taken about whether or not some breakdown of starch appeared to have happened.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:
9780134580999
Author:
Elaine N. Marieb, Katja N. Hoehn
Publisher:
PEARSON

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Anatomy & Physiology
Biology
ISBN:
9781259398629
Author:
McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:
Mcgraw Hill Education,

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:
9780134580999
Author:
Elaine N. Marieb, Katja N. Hoehn
Publisher:
PEARSON

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Anatomy & Physiology
Biology
ISBN:
9781259398629
Author:
McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:
Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:
9780815344322
Author:
Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:
W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:
9781260159363
Author:
Martin, Terry R., Prentice-craver, Cynthia
Publisher:
McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:
9781260231700
Author:
Sylvia S. Mader, Michael Windelspecht
Publisher:
McGraw Hill Education