How close are the best density values obtained for copper, iron, aluminum and glass compared to the actual values? If there is a discrepancy, try to explain why the values are different?
How close are the best density values obtained for copper, iron, aluminum and glass compared to the actual values? If there is a discrepancy, try to explain why the values are different?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter8: Molecules And Materials
Section: Chapter Questions
Problem 8.72PAE
Related questions
Question
I need help here please please
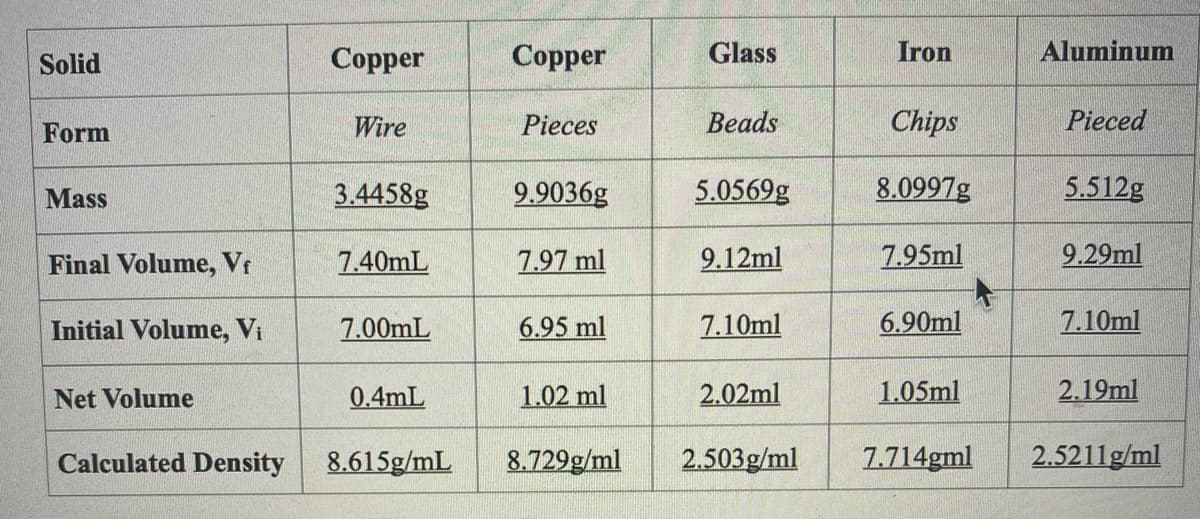
Transcribed Image Text:Solid
Соpper
Сopper
Glass
Iron
Aluminum
Form
Wire
Pieces
Вeads
Chips
Pieced
Mass
3.4458g
9.9036g
5.0569g
8.0997g
5.512g
Final Volume, Vr
7.40mL
7.97 ml
9.12ml
7.95ml
9.29ml
Initial Volume, Vi
7.00mL
6.95 ml
7.10ml
6.90ml
7.10ml
Net Volume
0.4mL
1.02 ml
2.02ml
1.05ml
2.19ml
Calculated Density
8.615g/mL
8.729g/ml
2.503g/ml
7.714gml
2.5211g/ml
Transcribed Image Text:ETECTAR IDIOMA
INGLÉS
ESPAÑOL
FRANCÉS
How close are the best density values obtained for copper, iron,
aluminum and glass compared to the actual values? If there is a
discrepancy, try to explain why the values are different? Y
<>
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax