Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter2: Chemical Compounds
Section: Chapter Questions
Problem 123QRT
Related questions
Question
how do i calculate the molarity of KMnO4. in the titration the buret was filled with 0.0815 M of KMnO4
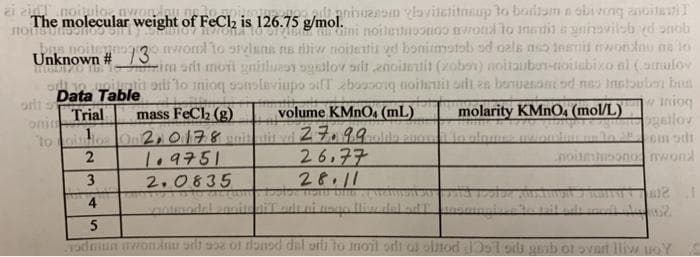
Transcribed Image Text:lt aniuanom vlovitetitnsup to borliam a abi ng anoitai
The molecular weight of FeCl2 is 126.75 g/mol.noitestuponoo mrotl to inetit a yninavilob ed snob
aini
noitenogo nwond to stvlans ns iliw noizentia vd bonimstob od oala no 1Ineis wonlau na to
rim sdt moti gnislun ogailov srit enoitetit (zobov) noitaubon-ioiabixo al (araulov
h ordi lo inioq poslaviupo silT zboooong noiluit ali 2a bonuasa od nas Instouben bna
w inioq
ogailov
Unknown # /3
Data Table
Trial
onim
to ilos On 2,0178
volume KMNO4 (mL)
27.99
molarity KMNO, (mol/L)
in alome
mass FeCl2 (g)
aum
26,77
28.11
noitensono nwond
T.9751
2.0835
3
Tadmun awonlnu sili soa o donsd dal orli to inoit odi ol olnod Do1 oil gab ot svart liv uoY
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning