University Physics Volume 3
17th Edition
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:William Moebs, Jeff Sanny
Chapter9: Condensed Matter Physics
Section: Chapter Questions
Problem 38CQ: What is the Meissner effect?
Related questions
Question
How do I use the mass (g) to determine what each cylinder is?
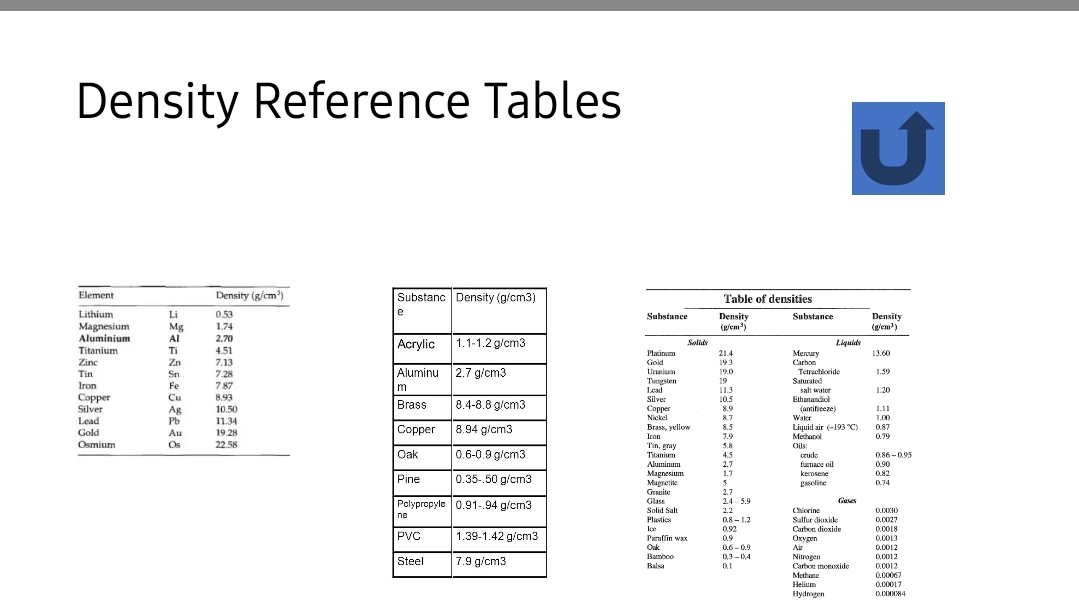
Transcribed Image Text:Density Reference Tables
Density (g/cm)
Substanc Density (g/cm3)
Element
Table of densities
Lithium
Li
0.53
e
Substance
Density
(g/em)
Substance
Density
(giem')
Magnesium
Aluminium
Mg
Al
1.74
2.70
Acrylic
1.1-1.2 g/cm3
Liguids
Solids
Titanium
Ti
4.51
Platinum
Gold
21.4
193
Meroury
Carbon
Tetrachioride
13.60
Zinc
Tin
Zn
7.13
Sn
Fe
Aluminu 2.7 g/cm3
Urunium
19.0
19
11.3
10.5
1.59
7.28
787
Tungsten
Lead
Silver
Saluraled
salt water
Ethanandiol
(antifreeze)
Iron
m
1.20
Copper
Silver
Lead
Cu
8.93
Brass
8.4-8.8 g/cm3
Ag
Pb
10.50
11.34
Copper
Nickel
Brass, yellow
Iron
Tin, gray
8.9
1.11
8.7
8.5
7.9
Wakr
1.00
Copper
8.94 g/cm3
Liquid air (-193 C
Methanol
0.87
0.79
Gold
Au
19.28
Osmium
Os
22.58
Oils:
zudo
furnace oil
kerosene
5.8
Oak
0.6-0.9 g/cm3
Titanium
Aluminum
4.5
2.7
1.7
0.86 -0.95
0.90
0.92
Magnesium
Magnetite
Granite
Pine
0.35-.50 g/cm3
gasoline
0.74
2.7
2.4 5.9
Gues
Polypropyle 0.91-.94 g/cm3
Glass
Solid Salt
22
Chiorine
Salfur dioxide
Carbon dioxide
na
0.8 - 1.2
0,92
0.9
0.6-0.9
Plastics
D.0027
le
0.0018
PVC
1.39-1.42 g/cm3
Paraffin wax
Ouk
Bamboo
Oxvpen
0,0013
0.0012
Air
0,3 -0,4
Nitrogen
Carbon monoxide
0,0012
Steel
7.9 g/cm3
Balsa
Methane
Helium
Hydrogen
0.0012
0.00067
0.00017
0.000M4

Transcribed Image Text:mm 1
5
8
9
10 11
12 13 14 15
IMWS 10OHDS
Made in China
089838
31
262.5g
9.7g
126.5g
66.6g
41.1g
Expert Solution
Step 1
With the help of rular, we have to measure the diameter(d) and height (h) of each cylinder.
Then we have to calculate the volume (V) of each cylinder.
Volume V = πr²h where r = d/2
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning