An Introduction to Physical Science
14th Edition
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Chapter5: Temperature And Heat
Section: Chapter Questions
Problem 31SA
Related questions
Question
How does the molar specific heat of a real gas at constant pressure differentiate from an ideal gas
Expert Solution
Step 1
Molar specific heat can be defined as the amount of heat needed to increase the temperature of 1 mole of the substance through 1 degree Celsius.
It is denoted by C
Specific heat capacity at constant volume (CV): it is defined as the amount of heat needed to increase the temperature of 1 g of gas through 1 degree Celsius at constant volume.
Specific heat capacity at constant pressure (CV): it is defined as the amount of heat needed to increase the temperature of 1 g of gas through 1 degree Celsius at constant pressure.
Step 2
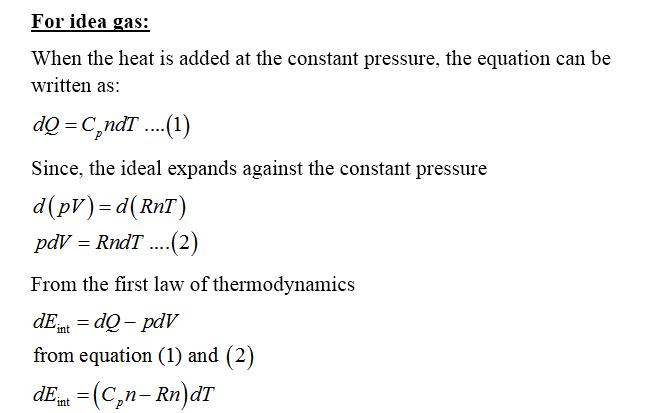
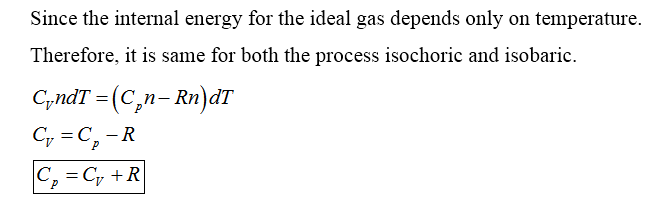
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning



An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning



Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning