How is covalent naming similar to ionic? What is different for covalent naming? Write the prefixes for each of the following 1: 2: 3: 4: 5: 6: What is the name or formula of the following: SCI, XeF (yea, Xe is odd..) Carbon Dioxide: Diphosphorus Pentoxide:
How is covalent naming similar to ionic? What is different for covalent naming? Write the prefixes for each of the following 1: 2: 3: 4: 5: 6: What is the name or formula of the following: SCI, XeF (yea, Xe is odd..) Carbon Dioxide: Diphosphorus Pentoxide:
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 86AP
Related questions
Question

Transcribed Image Text:How is covalent naming similar to ionic?
What is different for covalent naming?
Write the prefixes for each of the following
1:
2:
3:
4:
5:
6:
What is the name or formula of the following:
SCI,
XeF,
(yea, Xe is odd..)
Carbon Dioxide:
Diphosphorus Pentoxide:
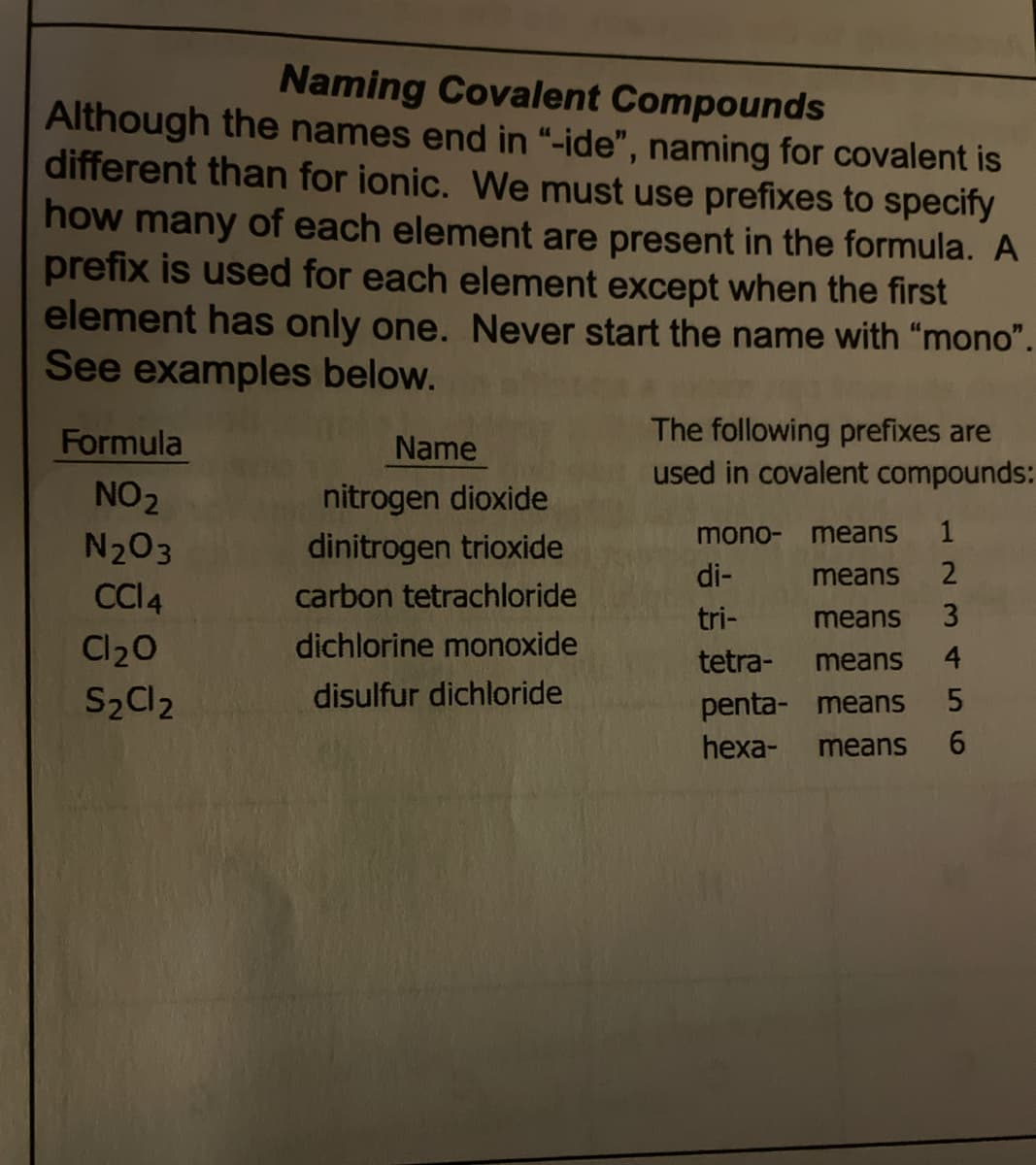
Transcribed Image Text:Naming Covalent Compounds
Although the names end in "-ide", naming for covalent is
different than for ionic. We must use prefixes to specify
how many of each element are present in the formula. A
prefix is used for each element except when the first
element has only one. Never start the name with "mono".
See examples below.
The following prefixes are
used in covalent compounds:
Formula
Name
nitrogen dioxide
dinitrogen trioxide
NO2
mono-
means
1
N203
di-
means
carbon tetrachloride
tri-
means
3
dichlorine monoxide
Cl20
S2C12
tetra-
means
4
disulfur dichloride
penta- means
hexa-
5
means
Expert Solution
Step 1
Given,
How is covalent naming similar to ionic naming ?
What is different in covalent naming ?
Name the prefixes = ?
What is the name and formula of the following ?
a). SCl5
b). XeF6
c). Carbon dioxide
d). Diphosphorus pentoxide
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning