Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter37: Qualitative Analysis Of Group Ii Cations
Section: Chapter Questions
Problem 3ASA
Related questions
Question
100%
How is the following reaction carried out ?
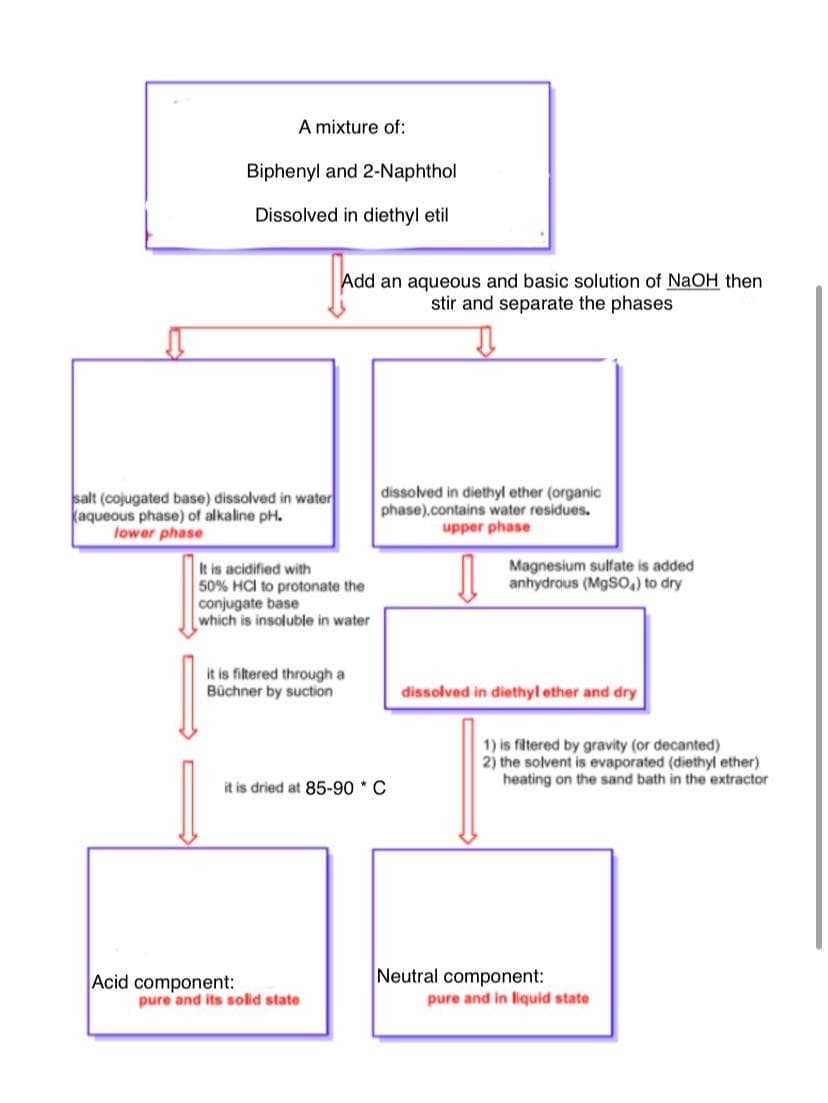
Transcribed Image Text:A mixture of:
Biphenyl and 2-Naphthol
Dissolved in diethyl etil
Add an aqueous and basic solution of NaOH then
stir and separate the phases
salt (cojugated base) dissolved in water
kaqueous phase) of alkaline pH.
lower phase
dissolved in diethyl ether (organic
phase).contains water residues.
upper phase
Magnesium sulfate is added
anhydrous (MgS) to dry
It is acidified with
50% HCI to protonate the
conjugate base
which is insoluble in water
it is filtered through a
Büchner by suction
dissolved in diethyl ether and dry
1) is filtered by gravity (or decanted)
2) the solvent is evaporated (diethyl ether)
heating on the sand bath in the extractor
it is dried at 85-90 * C
Acid component:
pure and its solid state
Neutral component:
pure and in lilquid state
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole