How many copper atoms are there per unit cell? (Show calculations.) What is the number of oxygens per unit cell? and Y? and Ba? What is the formula for this superconductor? The coppers in this unit cell occupy two different kinds of sites. Based on the number of each of these types and the formula for the compound, what are the oxidation states for copper in this compound?
How many copper atoms are there per unit cell? (Show calculations.) What is the number of oxygens per unit cell? and Y? and Ba? What is the formula for this superconductor? The coppers in this unit cell occupy two different kinds of sites. Based on the number of each of these types and the formula for the compound, what are the oxidation states for copper in this compound?
University Physics Volume 3
17th Edition
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:William Moebs, Jeff Sanny
Chapter9: Condensed Matter Physics
Section: Chapter Questions
Problem 10CQ: Describe the difference between a face-centered cubic structure (FCC) and a body-centered cubic...
Related questions
Question
How many copper atoms are there per unit cell? (Show calculations.) What is the number of oxygens per unit cell? and Y? and Ba? What is the formula for this superconductor? The coppers in this unit cell occupy two different kinds of sites. Based on the number of each of these types and the formula for the compound, what are the oxidation states for copper in this compound?
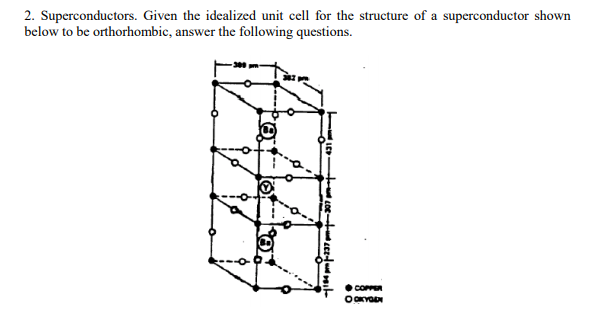
Transcribed Image Text:2. Superconductors. Given the idealized unit cell for the structure of a superconductor shown
below to be orthorhombic, answer the following questions.
• COER
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax