How many electrons does phosphorus have in its valence shel? (B) Three 1. (A) One (C) five (D) seven How many electrons does the element with an electron configuration of 1s 2s 2p have in its outermost shell? (A) two 2. (B) four (C) six (D) eight 3. In a double bond, how many electrons are shared between two atoms? (A) two (C) six (B) four (D) eight 4. In a polar covalent bond, the more electronegative atom acquires (A) 8+ charge (B) 8- charge (C) zero charge (D) Either A or B 5. Refer to Figure 1 shown on the right, which of the following statement is TRUE? (A) Br atom is more electronegative than Cl atom. (B) Clatom is more electronegative than Br atom. (C) Cl atom acquire a &+ charge. (D) Electrons move towards the Br atom. Br-Cl For Items 6 to 10, refer to Figure 2: Figure 1 Identify the hybridization state of the endrcled atom. (A) sp (B) sp 6. (C) sp' (D) s 7. Predict the bond angle around the encircled atom. (A) 0° (B) 109.5 (C) 120° (D) 180° :0: 8. Determine the formal charge of Oxygen atom. (A) -1 (B) 0 (C) +1 (D) 6 H-N-C=€C–H 9. The total number of a bonds H H (A) 4 (B) 5 (C) 6 (D) 7 Figure 2 10. The total number of m bonds (A) 0 (B) 1 (C) 2 (D) 3 11. Which of the following structural formulas has the molecular formula CaHi2 ? (A) 3 and 4 (B) 2 and 3 (C) 1,2 and 5 (D) 1, 4 and 5 26. Select the necessary reagents to convert: (A) H,O and H,So. (B) H,SO, and heat (C) BH, then H0, and NaOH (D) KMNO, and H,SO, H,C OH
How many electrons does phosphorus have in its valence shel? (B) Three 1. (A) One (C) five (D) seven How many electrons does the element with an electron configuration of 1s 2s 2p have in its outermost shell? (A) two 2. (B) four (C) six (D) eight 3. In a double bond, how many electrons are shared between two atoms? (A) two (C) six (B) four (D) eight 4. In a polar covalent bond, the more electronegative atom acquires (A) 8+ charge (B) 8- charge (C) zero charge (D) Either A or B 5. Refer to Figure 1 shown on the right, which of the following statement is TRUE? (A) Br atom is more electronegative than Cl atom. (B) Clatom is more electronegative than Br atom. (C) Cl atom acquire a &+ charge. (D) Electrons move towards the Br atom. Br-Cl For Items 6 to 10, refer to Figure 2: Figure 1 Identify the hybridization state of the endrcled atom. (A) sp (B) sp 6. (C) sp' (D) s 7. Predict the bond angle around the encircled atom. (A) 0° (B) 109.5 (C) 120° (D) 180° :0: 8. Determine the formal charge of Oxygen atom. (A) -1 (B) 0 (C) +1 (D) 6 H-N-C=€C–H 9. The total number of a bonds H H (A) 4 (B) 5 (C) 6 (D) 7 Figure 2 10. The total number of m bonds (A) 0 (B) 1 (C) 2 (D) 3 11. Which of the following structural formulas has the molecular formula CaHi2 ? (A) 3 and 4 (B) 2 and 3 (C) 1,2 and 5 (D) 1, 4 and 5 26. Select the necessary reagents to convert: (A) H,O and H,So. (B) H,SO, and heat (C) BH, then H0, and NaOH (D) KMNO, and H,SO, H,C OH
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter3: Chemical Bonds
Section: Chapter Questions
Problem 3.109P: 3-109 Until several years ago, the two chlorofluorocarbons (CFCs) most widely used as heat transfer...
Related questions
Question
Please answer the question below:
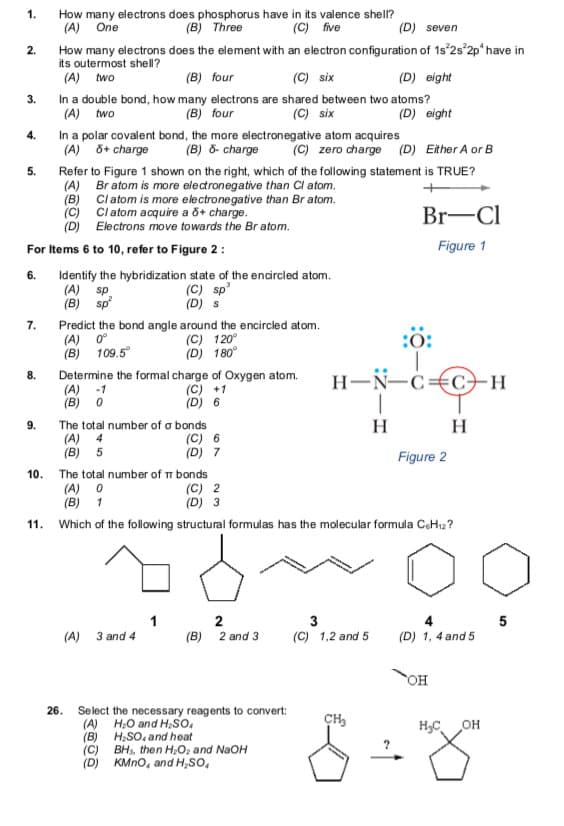
Transcribed Image Text:1.
How many electrons does phosphorus have in its valence shell?
(A) One
(B) Three
(C) five
(D) seven
How many electrons does the element with an electron configuration of 1s 2s 2p* have in
its outermost shell?
(A) two
2.
(B) four
(C) six
(D) eight
In a double bond, how many electrons are shared between two atoms?
(B) four
3.
(A) two
(C) six
(D) eight
In a polar covalent bond, the more electronegative atom acquires
(A) õ+ charge
4.
(B) ô- charge
(C) zero charge (D) Either A or B
Refer to Figure 1 shown on the right, which of the following statement is TRUE?
(A) Br atom is more electronegative than Cl atom.
(B) Cl atom is more electronegative than Br atom.
(c) Cl atom acquire a õ+ charge.
(D) Electrons move towards the Br atom.
5.
Br-Cl
For Items 6 to 10, refer to Figure 2 :
Figure 1
Identify the hybridization state of the encircled atom.
(A) sp.
(B) sp
6.
(C) sp'
(D) s
Predict the bond angle around the encircled atom.
(A) 0°
(B) 109.5°
7.
(C) 120°
(D) 180°
:0:
Determine the formal charge of Oxygen atom.
(A) -1
(B) 0
H-N-c€C+H
8.
(C) +1
(D) 6
H
The total number of a bonds
(A) 4
(B) 5
9.
H
(C) 6
(D) 7
Figure 2
The total number of m bonds
(A) O
(B) 1
10.
(C) 2
(D) 3
11.
Which of the following structural formulas has the molecular formula CeHı2?
1
2
(B) 2 and 3
3
5
(A)
3 and 4
(C) 1,2 and 5
(D) 1, 4 and 5
OH
26.
Select the necessary reagents to convert:
(A) H,O and H,so,
(B) H;SO, and heat
(C) BH, then H;O, and NaOH
(D) KMno, and H,so,
CH,
H,C
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning