How many gallons of gasoline must be burned in order to raise the temperature of 10 liters of water from 25o C to 50o C, assuming 50 percent efficiency in converting the heat released into heat in the water? (The heat of combustion of gasoline is 3.4 × 107 cal/gallon.)
How many gallons of gasoline must be burned in order to raise the temperature of 10 liters of water from 25o C to 50o C, assuming 50 percent efficiency in converting the heat released into heat in the water? (The heat of combustion of gasoline is 3.4 × 107 cal/gallon.)
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter6: Energy Of A System
Section: Chapter Questions
Problem 11CQ
Related questions
Question
18. How many gallons of gasoline must be burned in order to raise the temperature of 10 liters of water from 25o C to 50o C, assuming 50 percent efficiency in converting the heat released into heat in the water?
(The heat of combustion of gasoline is 3.4 × 107 cal/gallon.)
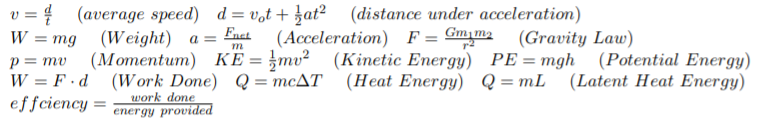
Transcribed Image Text:v = { (average speed) d = v,t + at? (distance under acceleration)
W %3D mg (Weight) а %3
Fact
(Acceleration) F= Gm,m2
(Gravity Law)
(Kinetic Energy) PE=mgh (Potential Energy)
p = mv
W = F ·d (Work Done) Q= mcAT (Heat Energy) Q=mL (Latent Heat Energy)
effciency =
%3D
work done
energy provided
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning