Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.65QE
Related questions
Question
Hello,
Need help for these two. Please show specific steps.
Thank you for your help!
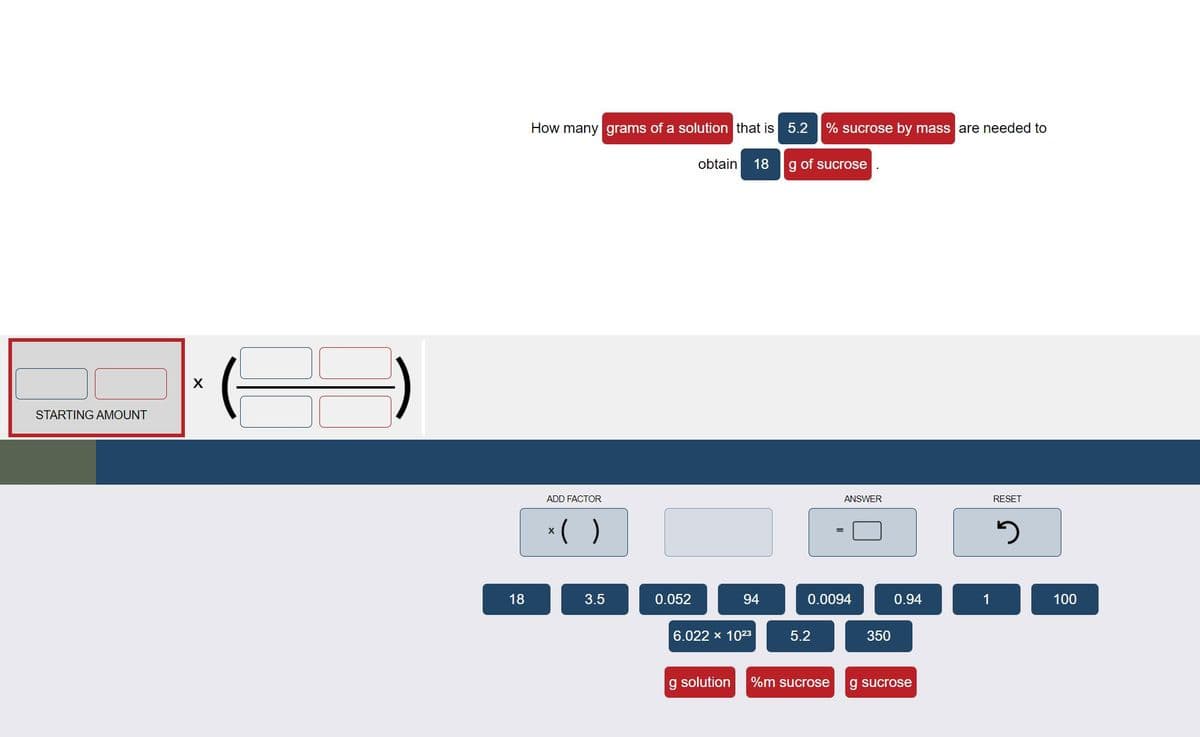
Transcribed Image Text:How many grams of a solution that is 5.2
% sucrose by mass are needed to
obtain
18
g of sucrose
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
18
3.5
0.052
94
0.0094
0.94
1
100
6.022 x 1023
5.2
350
g solution
%m sucrose
g sucrose
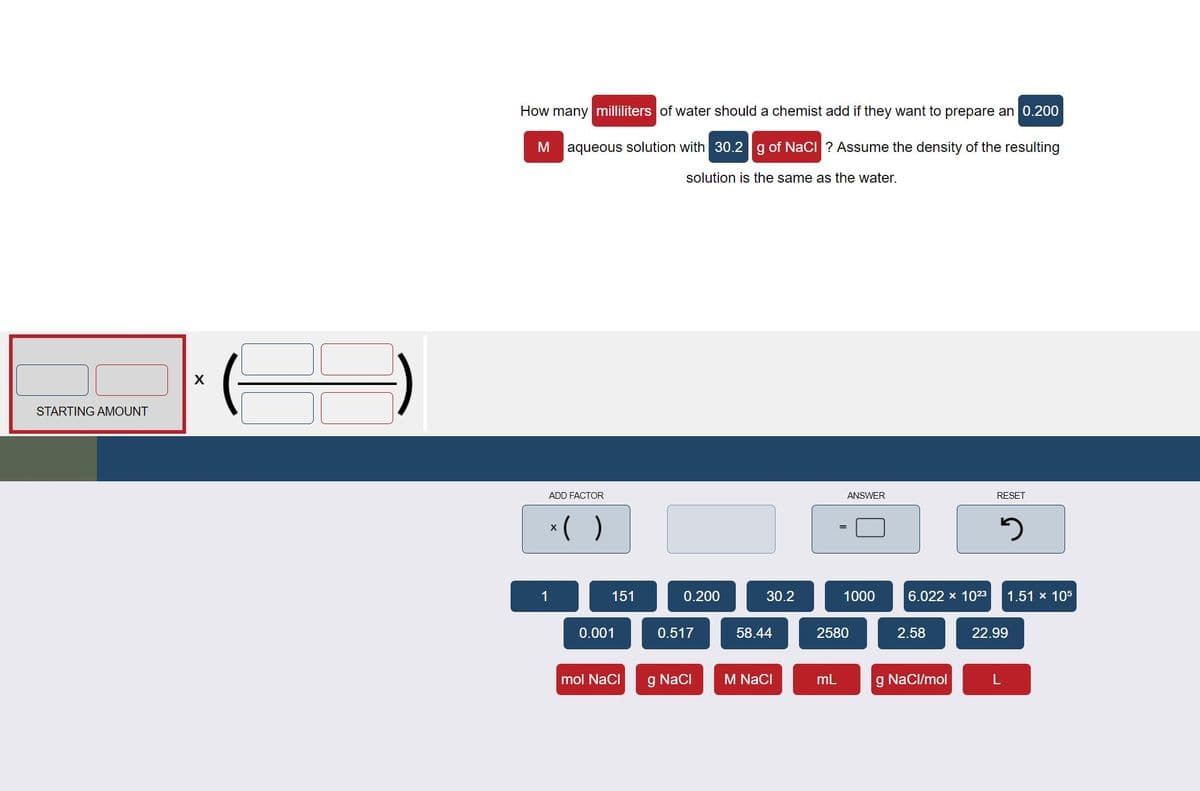
Transcribed Image Text:How many milliliters of water should a chemist add if they want to prepare an 0.200
aqueous solution with 30.2g of NaCI ? Assume the density of the resulting
solution is the same as the water.
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
1
151
0.200
30.2
1000
6.022 x 1023
1.51 x 105
0.001
0.517
58.44
2580
2.58
22.99
mol NaCi
g Naci
M NaCI
mL
g NaCl/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning