How many grams of Na solid are required to completely react with 19.2 L of Cl: gas at STP according to the following chemical reaction? Remember 1 mol of an ideal gas has a volume of 22.4 Lat STP.
How many grams of Na solid are required to completely react with 19.2 L of Cl: gas at STP according to the following chemical reaction? Remember 1 mol of an ideal gas has a volume of 22.4 Lat STP.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 100E: The oxides of Group 2A metals (symbolized by M here) react with carbon dioxide according to the...
Related questions
Question
Please refer to photo! Thanks!
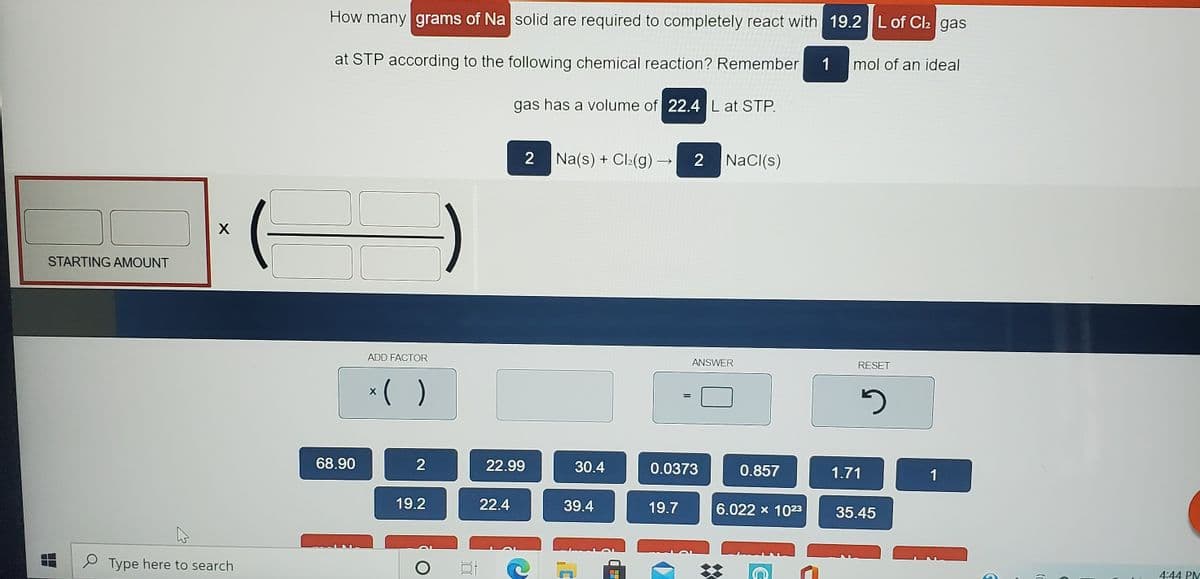
Transcribed Image Text:How many grams of Na solid are required to completely react with 19.2 L of Cl2 gas
at STP according to the following chemical reaction? Remember
1
mol of an ideal
gas has a volume of 22.4 L at STP.
Na(s) + Cl:(g) –
NaCI(s)
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
()
68.90
22.99
30.4
0.0373
0.857
1.71
1
19.2
22.4
39.4
19.7
6.022 x 1023
35.45
P Type here to search
4:44 PM
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning