How many liters of 0.305 MK Po, solution are necessary to completely react with 187 mL of 0.0184 M NICI, according to the balanced chemical reaction: 2 KPO,(aq) + 3 NICI (aq) – Ni,(PO,),(s) + 6 KCI(aq)
How many liters of 0.305 MK Po, solution are necessary to completely react with 187 mL of 0.0184 M NICI, according to the balanced chemical reaction: 2 KPO,(aq) + 3 NICI (aq) – Ni,(PO,),(s) + 6 KCI(aq)
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 14.97QE: According to the Resource Conservation and Recovery Act (RCRA), waste material is classified as...
Related questions
Question
I need the whole equation layout not just the final result. I need a complete equation with units please.
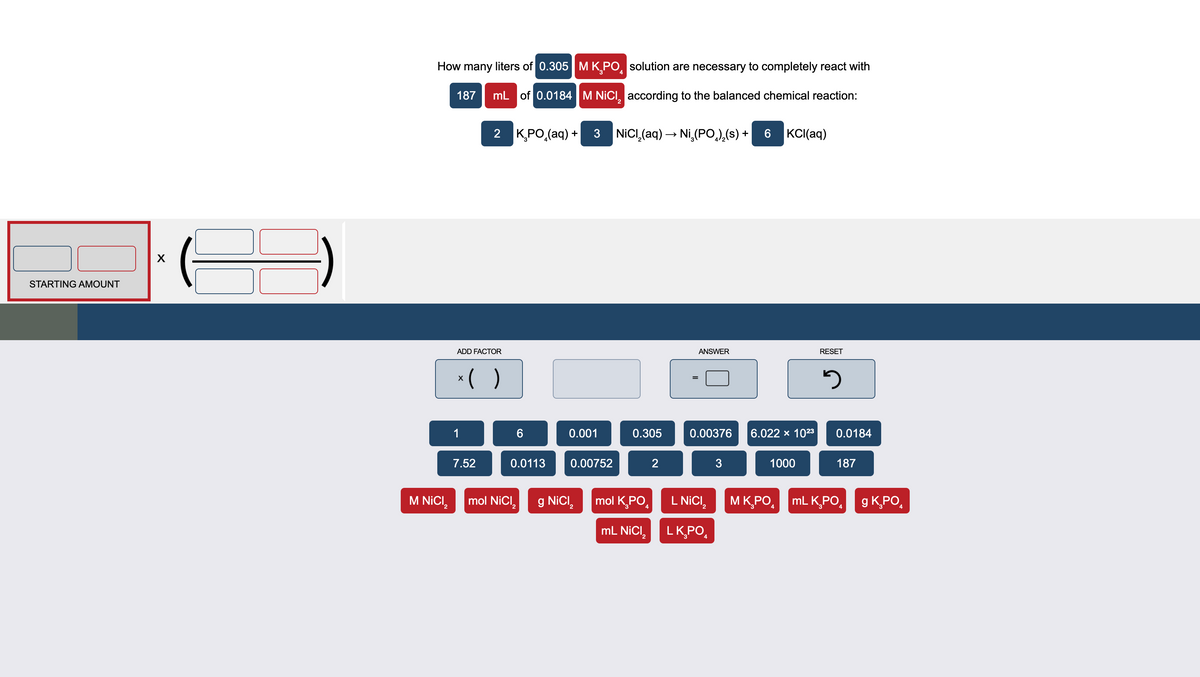
Transcribed Image Text:How many liters of 0.305 | M K PO, solution are necessary to completely react with
4
187
mL of 0.0184 M NICI, according to the balanced chemical reaction:
2 KPO,(aq) + 3 NICI,(aq) → Ni,(PO,),(s) + 6 KC(aq)
KCI(aq)
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
1
6
0.001
0.305
0.00376
6.022 x 1023
0.0184
7.52
0.0113
0.00752
2
3
1000
187
M NICI,
mol NiCI,
g NICI,
mol K,PO,
L NICI,
M K,PO,
mL K,PO,
g KPO,
2
4
mL NICI,
LKPO,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT