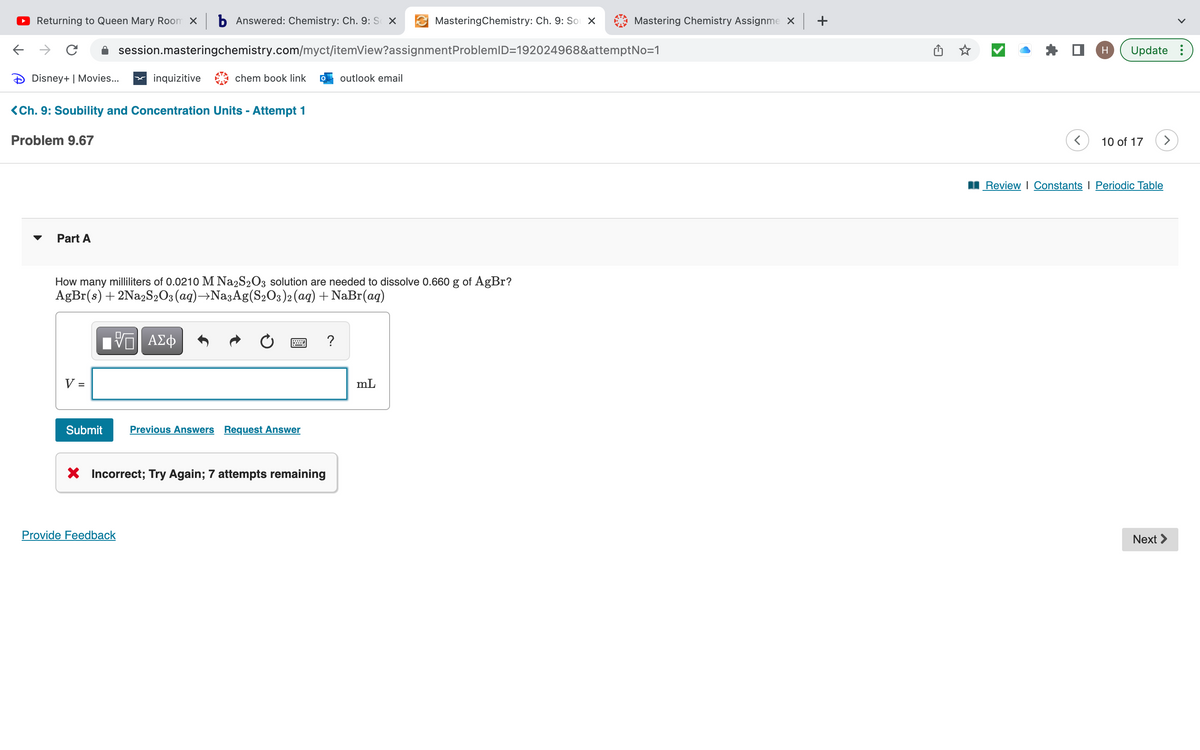
How many milliliters of 0.0210 M Na2S2O3 solution are needed to dissolve 0.660 g of AgBr? AgBr(s) + 2Na2S₂O3(aq) →NasAg(S₂O3)2 (aq) + NaBr(aq) 15. ΑΣΦ V = Submit BAC ? Previous Answers Request Answer mL
How many milliliters of 0.0210 M Na2S2O3 solution are needed to dissolve 0.660 g of AgBr? AgBr(s) + 2Na2S₂O3(aq) →NasAg(S₂O3)2 (aq) + NaBr(aq) 15. ΑΣΦ V = Submit BAC ? Previous Answers Request Answer mL
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 14Q
Related questions
Question
Transcribed Image Text:←
Returning to Queen Mary Room × b Answered: Chemistry: Ch. 9: SCX
с
Disney+ | Movies...
Part A
session.masteringchemistry.com/myct/itemView?assignment
<Ch. 9: Soubility and Concentration Units - Attempt 1
Problem 9.67
V =
inquizitive
chem book link
Provide Feedback
How many milliliters of 0.0210 M Na2S2O3 solution are needed to dissolve 0.660 g of AgBr?
AgBr(s) + 2Na2S2O3(aq) →Na3Ag(S₂O3)2 (aq) + NaBr(aq)
[ΨΕΙ ΑΣΦ
Submit Previous Answers Request Answer
X Incorrect; Try Again; 7 attempts remaining
outlook email
?
MasteringChemistry: Ch. 9: Sol X
ProblemID=192024968&attemptNo=1
mL
Mastering Chemistry Assignme × | +
H Update:
10 of 17
Review | Constants I Periodic Table
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning