How many moles of aluminum are required to completely react with 107 mL of 6.00 M H.SO. according to the balanced chemical reaction: 2 Al(s) + 3 H:SO.(aq) → Al:(SO.)»(aq) + 3 H:(g)
How many moles of aluminum are required to completely react with 107 mL of 6.00 M H.SO. according to the balanced chemical reaction: 2 Al(s) + 3 H:SO.(aq) → Al:(SO.)»(aq) + 3 H:(g)
Chapter5: Gases
Section: Chapter Questions
Problem 161IP: In the presence of nitric acid, UO2+ undergoes a redox process. It is converted to UO22+ and nitric...
Related questions
Question
Hello,
Need help for these two questions, please! Please show specific steps of solution.
Thank you for your support!
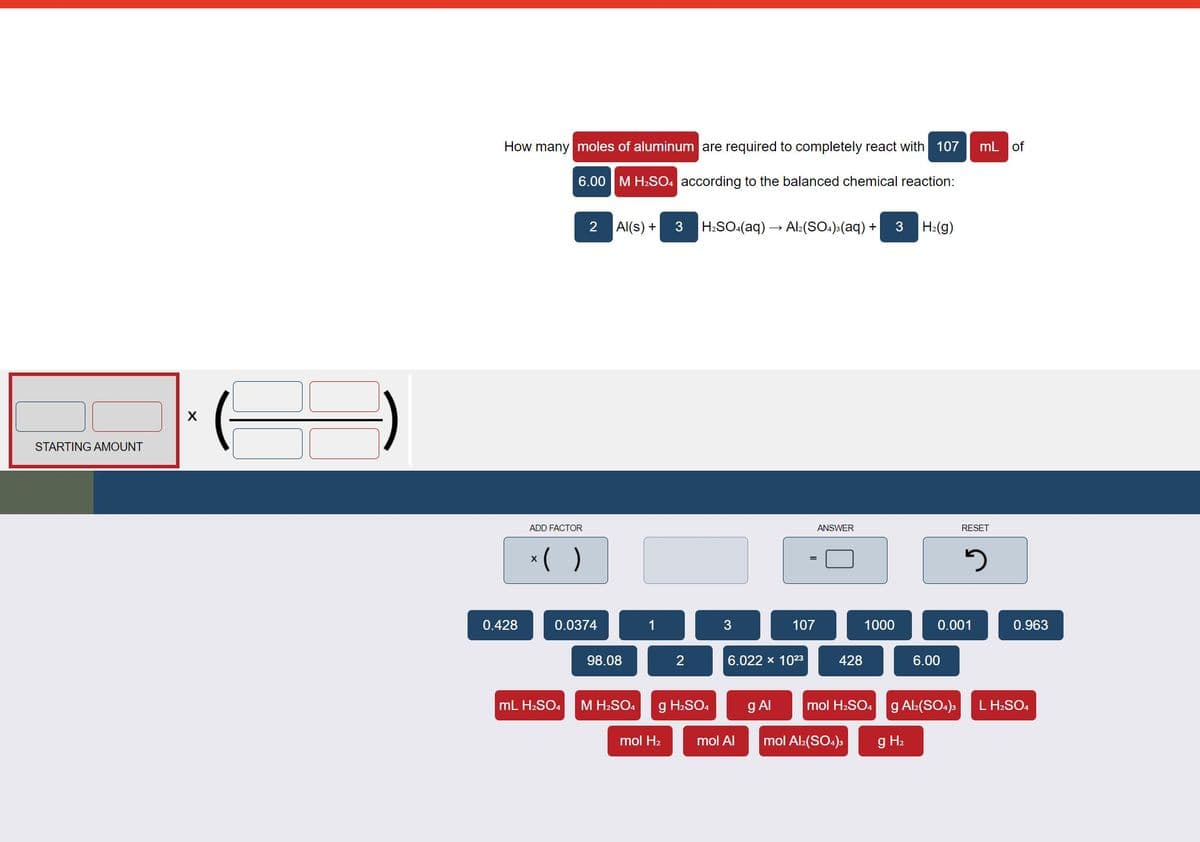
Transcribed Image Text:How many moles of aluminum are required to completely react with 107
mL of
6.00 M H2SO. according to the balanced chemical reaction:
2
Al(s) +
3
H:SO:(aq) → Al:(SO.)>(aq) +
H:(g)
X
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
0.428
0.0374
1
107
1000
0.001
0.963
98.08
6.022 x 1023
428
6.00
mL H2SO4
M H2SO4
g H2SO4
g AI
mol H2SO. g A:(SO4)3
L H2SO4
mol H2
mol Al
mol Al:(SO4)s
g H2
2.
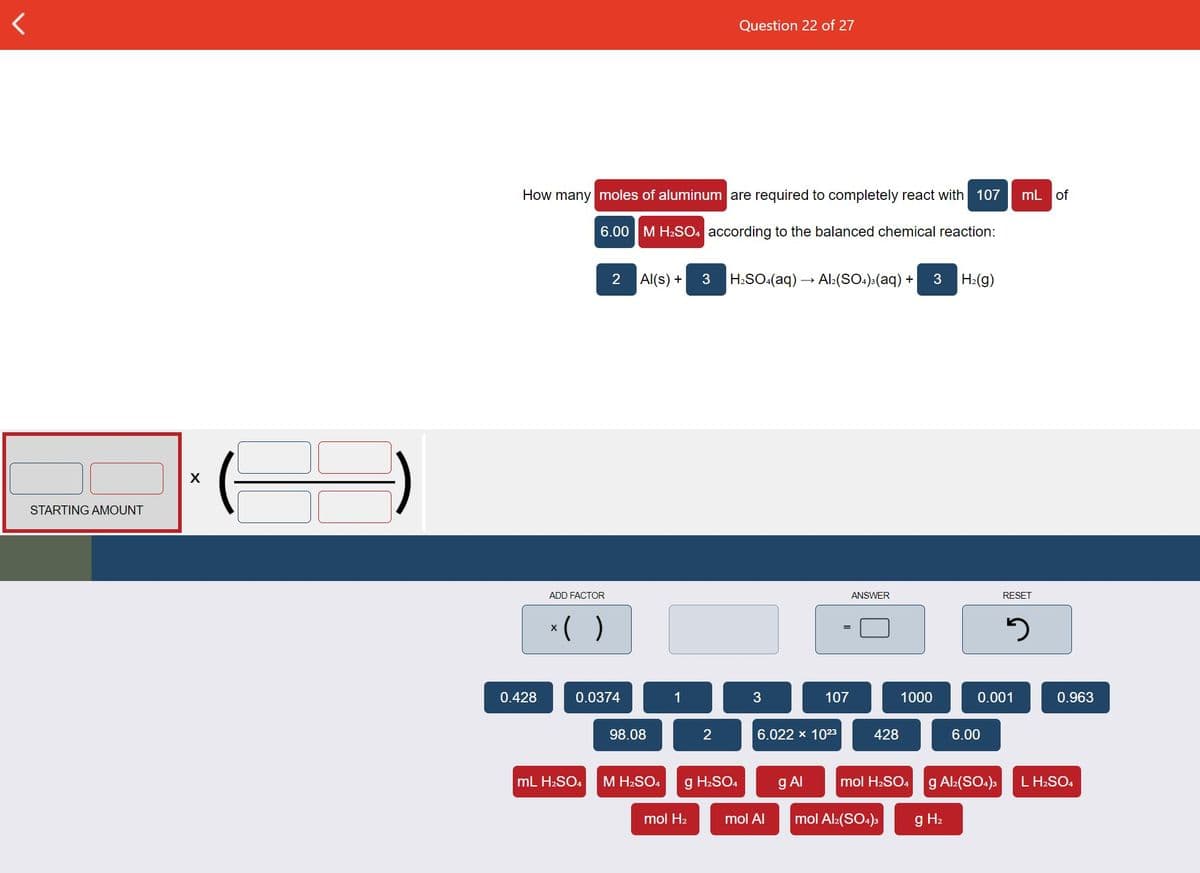
Transcribed Image Text:Question 22 of 27
How many moles of aluminum are required to completely react with 107
mL of
6.00
M H2SO4 according to the balanced chemical reaction:
Al(s) +
3
H:SO:(aq) – Al:(SO.)>(aq) +
H:(g)
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
0.428
0.0374
1
3
107
1000
0.001
0.963
98.08
6.022 x 1023
428
6.00
mL H2SO4
M H2SO4
g H2SO4
g AI
mol H2SO4 g Al:(SO4)3
L H2SO4
mol H2
mol Al
mol Al:(SO.)s
g H2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning