Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter5: Stoichiometry
Section: Chapter Questions
Problem 56E: What mass of compound is present in 5.00 moles of each of the compounds in Exercise 52?
Related questions
Question
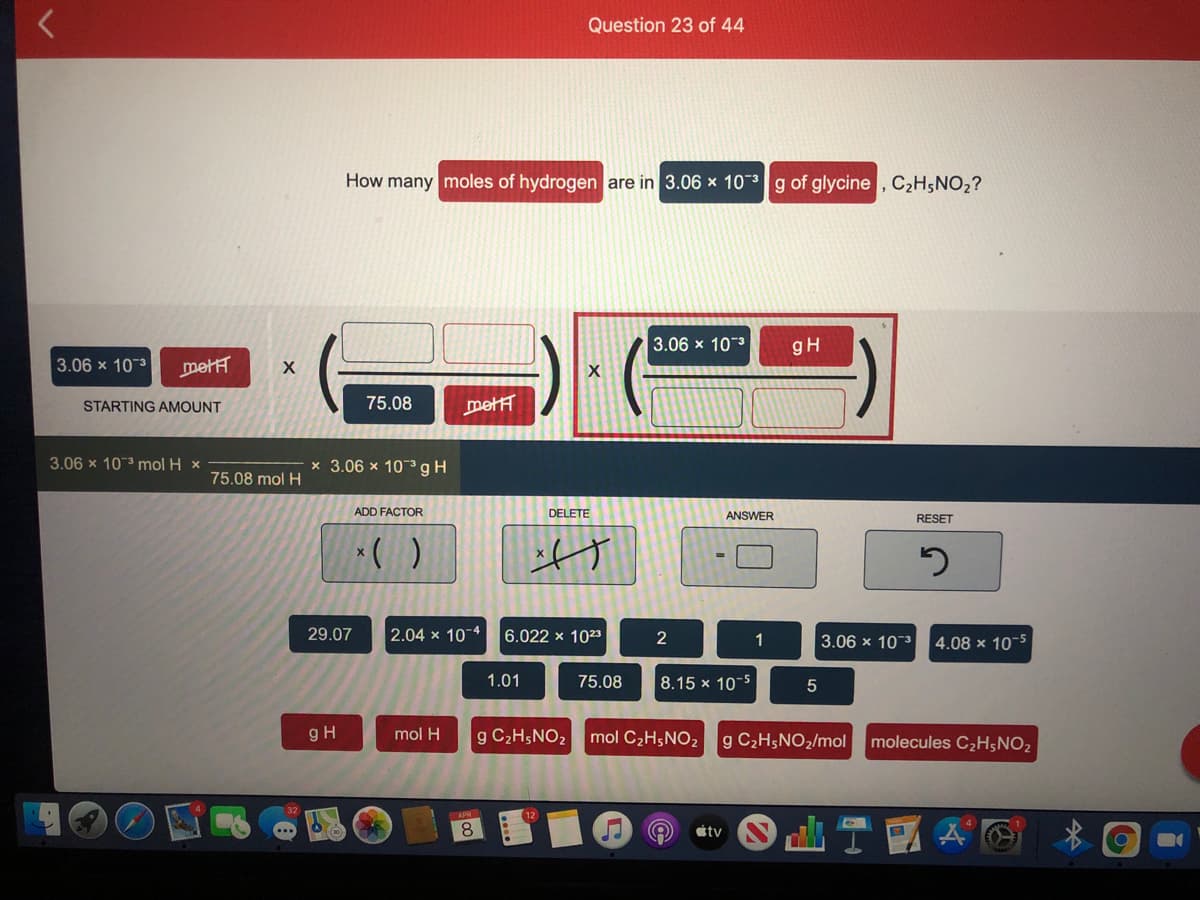
Transcribed Image Text:Question 23 of 44
How many moles of hydrogen are in 3.06 x 10-g of glycine , C2H;NO2?
3.06 x 10-3
gH
3.06 x 10 3
metH
STARTING AMOUNT
75.08
mett
3.06 x 103 mol H x
x 3.06 x 103gH
75.08 mol H
ADD FACTOR
DELETE
ANSWER
RESET
29.07
2.04 x 10 4
6.022 x 1023
2
1
3.06 x 103
4.08 x 10-5
1.01
75.08
8.15 x 10-5
gH
mol H
g C2H5NO2
mol C2H5NO2
g C2H5NO2/mol
molecules C2H;NO2
8
átv
Transcribed Image Text:History
Bookmarks
People
Tab
Window
Help
21%
O Chem101
G Determine t x
C How Many N
D (467) How t X
C Determine
M Application x
Technical E
à app.101edu.co
Update :
E Apps
M Gmail
Мaps
O YouTube
O Translate
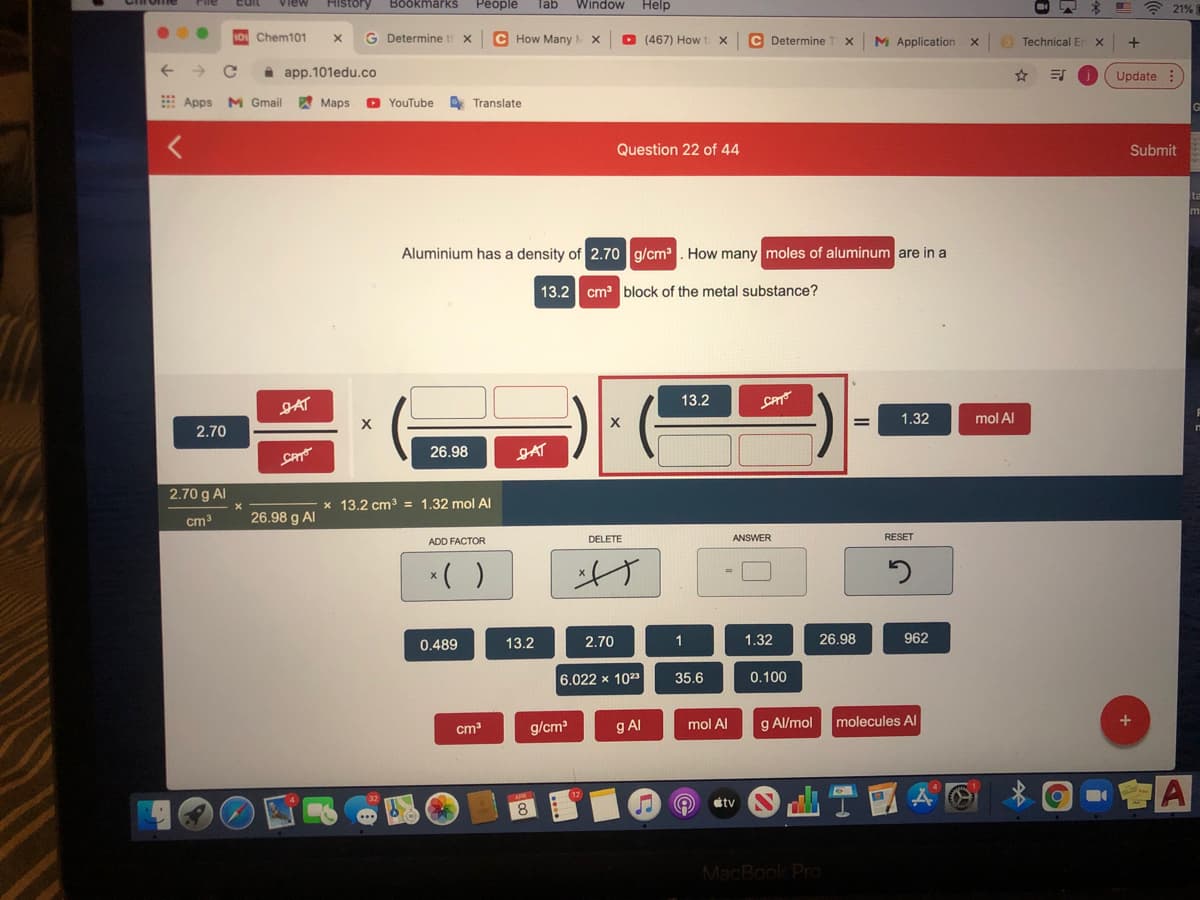
Question 22 of 44
Submit
Aluminium has a density of 2.70 g/cm. How many moles of aluminum are in a
13.2 cm block of the metal substance?
JAT
13.2
CAT
1.32
mol Al
2.70
26.98
JAT
CAT
2.70 g AI
x 13.2 cm3 = 1.32 mol Al
cm3
26.98 g Al
DELETE
ANSWER
RESET
ADD FACTOR
*( )
げ
0.489
13.2
2.70
1
1.32
26.98
962
6.022 x 102
35.6
0.100
g/cm
g Al
g Al/mol
molecules Al
cm3
mol Al
étv
MacBook Pro
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co