Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 51QAP: For each of the following samples of ionic substances, calculate the number of moles and mass of the...
Related questions
Question
Transcribed Image Text:Chrome
File
Edit
View
History
Bookmarks
Profiles
Tab
Window
Help
$ My Sac State | Sacramento Sta X
CHEM6A Intro General Chem
101 Chem101
A app.101edu.co
Update
y.
s E式
Question 15 of 32
Submi
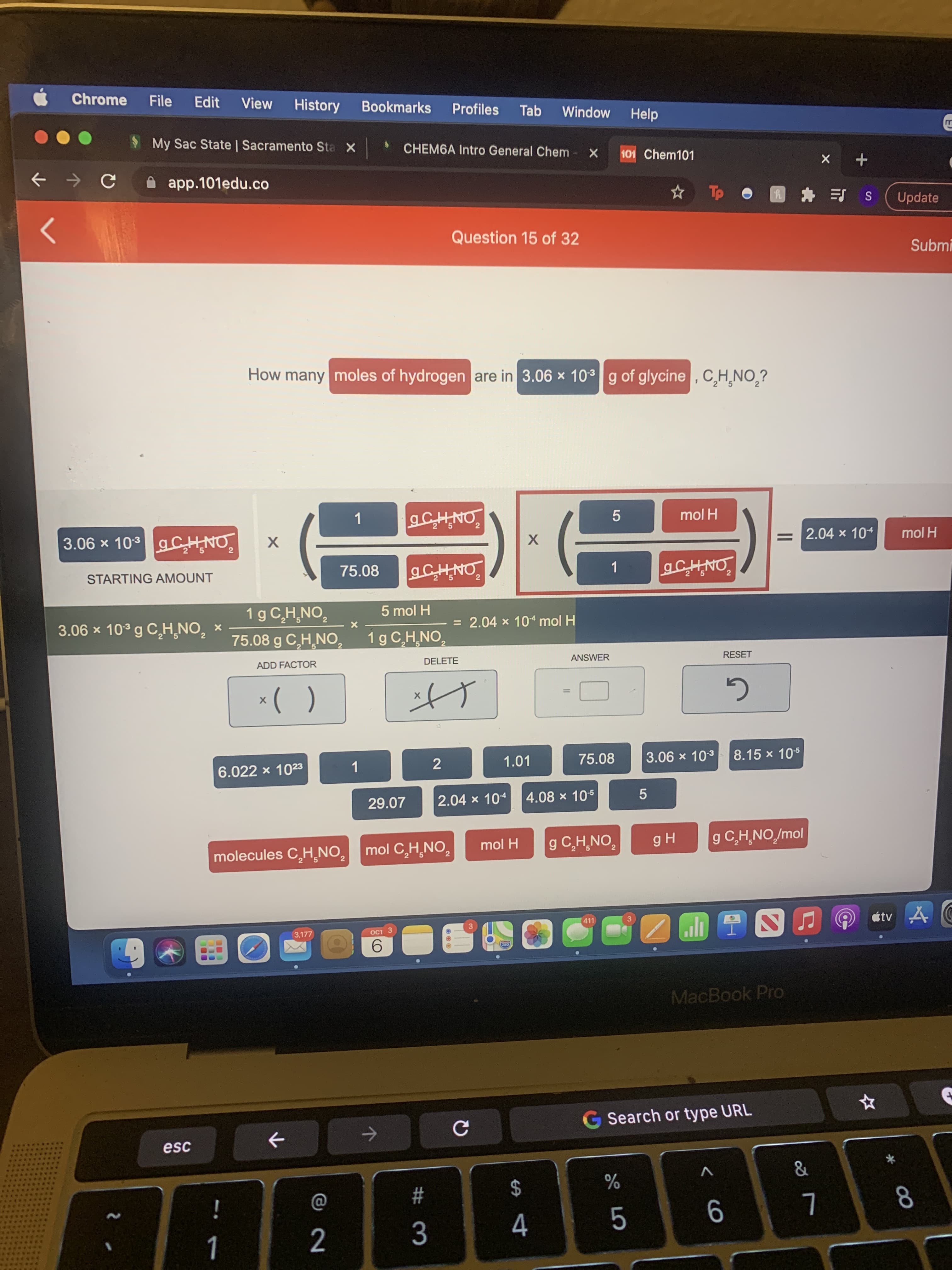
How many moles of hydrogen are in 3.06 x 103g of glycine , C,H¸NO,?
1
mol H
ONHJ 0L × 90'E
75.08
2.04 x 104
mol H
X
STARTING AMOUNT
1 g C,H,NO,
5 mol H
5.
3.06 x 10° g C,H,NO, x
= 2.04 x 10“ mol H
75.08 g C,H,NO,
1g C,H,NO,
DELETE
ANSWER
RESET
ADD FACTOR
%3D
( )
1.01
75.08
3.06 x 103
8.15 x 105
6.022 x 1023
1.
29.07
2.04 x 104
4.08 x 105
5.
mol H
molecules C,H̟NO, mol C,H NO,
joufON'Hɔ 6
ON'H'o 6
411
3,177
OCT 3
6.
MacBook Pro
G Search or type URL
esc
->
V
%
23
2$
L
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
