How many moles of NaOCl would be present in 1 gallon of commercial bleach (density = 1.05 g/ml), which is 6.25% mass percent active ingredient.
Q: II. For the alcohols below, propose at least two syntheses starting from compounds with fewer carbon…
A: To prepare the given alcohols with compounds with fewer carbon atoms than the product. To increase…
Q: 2. a) Explain what structural feature of the double bond is required for an alkene to exhibit…
A: The objective of the question is to find the structural features of the double bond for an alkene to…
Q: How many signals would you expect to find in the proton-decoupled 13C spectra of the following…
A:
Q: 3. Name the following alkynes. (a) (b) CH3CH₂CH₂CH₂CH₂CH₂C = CH CH₁ CH₁ CH₂-CH-CH-CH-CH—C—CH I CH₂…
A: The alkynes bear a triple bond between two carbon atoms. According to the IUPAC nomenclature, the…
Q: Provide the principle organic product(s) for the following reactions. If more than one product is…
A: The objective of this question is to identify the major products in the given reaction.
Q: A 50.00 ml aliquot of a solution containing Ca2+ and Mg2+ was buffered at pH 10 and titrated with…
A: The objective of the question is to determine the concentration of Ca2+ and Mg2+ in the solution.…
Q: Consider this organic reaction: Br CH 3 OH
A: Given is an alkyl halide and a nucleophile .To find the major product of the reaction.We consider…
Q: A solution is prepared by dissolving 371 g of sucrose (C₁2H22011) in 569 g of water. What is the…
A:
Q: Which molecule or ion do you think is most likely to act as a Lewis base? Hint: Think about the…
A:
Q: The following table provides some information on carbon dioxide solubility in water. C P k T (mol/L)…
A:
Q: Percent Yield
A: The objective of the question is to calculate the percent yield of the reaction based on the actual…
Q: 17) A reaction has an activation energy of 85 kJ. What percentage increase would one get for & for…
A:
Q: 4-methyl benzyl alcohol OH H
A: PCC is used to convert primary alcohol (1°) to aldehyde and secondary alcohol (2°) to ketone.
Q: Draw the products of the two step reaction sequence shown below. Ignore inorganic byproducts. mCPBA…
A: Opening of epoxide is regioselective reaction. The opening of epoxide depends upon the reaction…
Q: Interactive 3D display mode H₂ H₂C= H C CH₂ CH3 Spell out the full name of the compound.
A: • Select the longest carbon chain.• Number the carbon chain from one end in which the nearest…
Q: or a gaseous sample, Pressure (P) and quantity (n) are O inversely related O directly related O No…
A: The ideal gas is defined as that relates pressure, volume, quantity of gas, and absolute…
Q: For the reaction A (g) → 2 B (g), K = 14.7 at 298 K. What is the value of Q for this reaction at 298…
A: The reaction given is, A(g) → 2B(g) K = 14.7 at 298 K ∆G = -19.4 kJ/mol = -19.4 J/molWe…
Q: How can 1500.0 mL of 2.3 M aqueous H₂SO4 be made from concentrated sulfuric acid (18 M)?
A: The objective is to determine how the given amount of aqueous H2SO4 be made from the concentrated…
Q: Translate each word equation into a balanced chemical euqation, including phyical states for all…
A: To solve this problem, we have to write the balance equation for the given word equation.
Q: Carry out the following synthesis, using any necessary reagents. Show any synthetic intermediates.…
A: The objective of the question is to predict the reagents and steps followed to form the product…
Q: Rank these compounds in order of increasing basicity (most basic at the bottom). C A B Drag and drop…
A: Basicity means the ability of an atom to donate electrons. More the electron density on atom, more…
Q: In one reaction, 41.2 g of Cu(NO3)2 is produced. What amount (in mol) of CuCl2 was consumed? What…
A: AgNO3 + CuCl2 → AgCl + Cu(NO3)2Balancing the above equation, we get2AgNO3 + CuCl2 → 2AgCl +…
Q: Propose a synthesis for the following compound: A 1) 03; 2) DMS D The target molecule above can be…
A: We can convert acetylene to the given epoxide by using the reagents provided in the question. At…
Q: Draw the product of the reaction shown below. Ignore inorganic byproducts. 1. NaBH4 2. H3O+
A: The aim is to determine the product of the given reaction. Aldehydes and ketones in presence of…
Q: The following reaction does not produce the desired product but does produce a product that is a…
A:
Q: Draw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry…
A:
Q: Assign the correct stereochemical descriptor to each of these compounds: HO 1 Select one: O Compound…
A:
Q: The graphs below show sample data for reactant A. All the other reactants are in excess. Identify…
A: For first-order reactions, The integrated rate equation is,[A] = [A]0 - ktln[A] = - kt + ln[A]0 It…
Q: 4.71 trans-1,3-Dichlorocyclobutane has a measurable dipole moment. Explain why the individual dipole…
A: The objective of the question is to give reason why trans-1,3-dichlorocyclobutane, despite its trans…
Q: At a certain temperature the vapor pressure of pure acetyl bromide (CH₂COBr) is measured to be 0.68…
A: Information about the question
Q: 9. Show the characteristic fragmentation of the following ketones: a. b.
A: The objective of the question is to find the characteristic fragmentation of the given ketones.…
Q: Stereochemistry of hydroboration. There are two products that result from this reaction. Products 1…
A: The reaction adds water across the double bond and transforms alkenes into alcohols. The reaction…
Q: The hydrolysis of sucrose (C12H22O11) into glucose and fructose in acidic water has a rate constant…
A:
Q: A mixture of nitrogen and methane gases, in a 7.98 L flask at 48 °C, contains 9.27 grams of nitrogen…
A: An ideal gas is a hypothetical gas that has no intermolecular force and has negligible mass.An ideal…
Q: A mechanism consists of the following two elementary steps: (1) A₂ + 2BC (2) C2AB What is the…
A: Given MechanismTo determine:Molecularity of the first elementary step
Q: 2C1₂0, (g) →2Cl₂ (g) +50₂ (g) He fills a reaction vessel with Cl₂O, and measures its concentration…
A: The objective is to determine the rate law and rate constant.Given TIME 0s…
Q: Assign 1,2 and 3 configuration S or R
A: The following rules are used to assign the R/S configurations to the chiral centers.Identify chiral…
Q: Starting from the wedge-and-dash structure below (sighting down the indicated bond). Rotate only the…
A: Given compound:To determine:Eclipsed conformer of the structure by rotating the back carbon
Q: Draw the skeletal structure of the disulfide formed when the thiol shown is oxidized. SH
A: A skeletal structure in chemistry is a simplified representation of a molecule that conveys the…
Q: What effect does modification of the side chain of the following lead compound have in the modified…
A: Given is the structure of a lead molecule. It is modified to form a new molecule. What is the effect…
Q: 2. For a solution in which μ = 6.5 x 10-2, calculate K'p for н a. AgSCN.
A: Given salts u = 6.5 × 10-²a. AgSCNb. La(IO3)3c. PbI2d. MgNH4PO4 We have to find Ksp
Q: f.) g.) h.) ОН ОН PCC Buz SnH AIBN NalO4
A: Given some organic reactants and reagents. Determine the major products.
Q: Suppose a 250. mL flask is filled with 0.70 mol of NO₂, 0.20 mol of NO and 1.9 mol of CO₂. The…
A: Answer:For any reaction, value of equilibrium constant is equal to the ratio of molar concentration…
Q: Classify the following substituents according to whether they are electron donors or electron…
A: 1) Ph-S+(CH3)-:It show positive inductive effect because Sulphur having positive fromal charge…
Q: (1) P grapirepге: TN P TN
A:
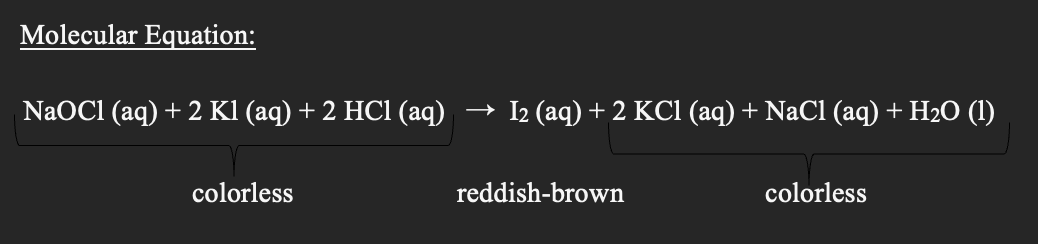
Q: lodine is sparingly soluble in pure water. However, it does 'dissolve' in solutions containing…
A: The equilibrium constant (Keq) of a reaction is the ratio of the product of the molar concentration…
Q: The equilibrium constant, Kc, for the following reaction is 6.93 x 104 at 438 K. PC15 (9)PC13 (9) +…
A: “Since you have posted multiple questions, we will provide the solution only to the first question…
Q: Reaction Scheme: CHO 8.80 NO₂ 6 5 L-tryptophan H₂O OH 7 NO₂
A: Tryptophan has one NH2 group that will generate imine intermediate and another basic N-atom that…
Q: PROBLEM Give IUPAC names for the following compounds. 4-28 (a) CH3 (b) (c) (e) H3C H3C. н- CH3 CH3…
A: The objective of the question is to give the names of the given compounds.
Q: Which of the indicated protons in the following compound would appear most upfield in the ¹H NMR…
A: NMR spectroscopy is a very important tool for the determination of the structure of the organic…
How many moles of NaOCl would be present in 1 gallon of commercial bleach (density = 1.05 g/ml), which is 6.25% mass percent active ingredient. (1 L = 1.057 qt.)
Step by step
Solved in 5 steps with 6 images

- You have 1.00 g of aluminum hydroxide in 100.0 mL of water. What is the concentration of both ions in solution? Ksp (Al(OH)3) = 1.8×10^–33 Please type answer note write by hend.What is the coefficient of Fe3+ when the following equation is balanced? CN− + Fe3+ → CNO− + Fe2+ (basic solution) Group of answer choices 1 2 3 4 5Which of the following reactions are redox reactions? Select all that apply. Group of answer choices None are redox reactions 11H+(aq) + 2MnO4–(aq) + 5Cl–(aq) --> 2Mn2+(aq) + 5HClO(aq) + 3H2O(l) CH3COOH(aq) + NaOH(aq) --> CH3COONa(aq) + H2O(l) 2Al(s) + 6H+(aq) --> 2Al3+(aq) + 3H2(g) 3AgNO3(aq) + FeCl3(aq) --> 3AgCl(s) + Fe(NO3)3(aq)
- Question: - Consider the following data for the titration of a vinegar solution by sodium hydroxide (NaOH) * Equation of the reaction: NaOH + CH3CO2H -> CH3CO2Na + NaCl * Initial NaOH level in a burette: 12.7 mL * Final NaOH level in the burette: (2.33x10^1) mL * Molarity of the NaOH solution: (4.3130x10^-1) mol/L * Volume of vinegar solution titrated: 10.0 mL Whaht is the molarity of the vinegar solution titrated?Balanced net ionic equation for: 1. CrO42- and 0.2M Ba(NO3)2 2. CrO42- and 0.2 M Pb (NO3)2 3. CrO42- and 0.2 M Fe (NO3)3 4. CrO42- and 0.2 M AgNO3Lactic acid is what causes the muscles the feeling of soreness or fatigue. In vitro study was done to investigate its properties and a lab assistant was tasked to prepare 1.8g of lactic acid dissolved in 200mL water yielded an ionization of 3.7%. What was the laboratory value of Ka of the lactic acid if its molar mass is 90 grams per mole?
- Select ALL the correct net ionic equations 2AgNO3 (aq)+ Na2CO3 (aq)--> 2NaNO3 (aq)+ Ag2CO3 (s) 2Ag+ (aq) + S2- (aq)--> Ag2S (s) Ag+ (aq) + OH- (aq)--> AgOH (s) 2Ag+(aq) + 2NO3- (aq) + 2Na+ (aq)+ SO42- (aq)--> 2Na+ (aq)+ 2NO3- (aq)+ Ag2SO4 (s)Need help with question 7 but 4-6 are here for context, thank you. Question 4 A standardized iron (II) solution that is 0.224 M in Fe2+ was used to determine the concentration of permanganate in solution. If the titration required 31.2 mL of Fe2+, how many moles of permanganate ion are present in solution? The pertinent balanced chemical equation is: MnO4- (aq) + 5 Fe2+ (aq) + 8 H+ (aq) --> Mn2+ (aq) + 5 Fe3+ (aq) + 4 H2O (l). Question 5 A standardized iron (II) solution that is 0.224 M in Fe2+ was used to determine the concentration of permanganate in solution. If the titration required 31.2 mL of Fe2+, how many moles of permanganate ion are present in solution? The pertinent balanced chemical equation is: MnO4- (aq) + 5 Fe2+ (aq) + 8 H+ (aq) --> Mn2+ (aq) + 5 Fe3+ (aq) + 4 H2O (l). Question 6 An ore containing solid titanium was processed so that it would yield titanium (IV) permanganate. A titration with an iron (II) standard was performed that indicated that…Net Ionic Equation for NaI + AgNO3
- Balance the following equations in basic solution. MnO4-(aq) + CN-(aq) --> MnO2(s) + OCN-(aq) S(s) + OCl-(aq) --> SO32-(aq) + Cl-(aq)What is the Net ionic equation of HNO3 and Na2SO3?Earth's oceans have a depth of 3800. m, a total area of 3.63 x 108km2, and an average concentration of dissolved gold ions of 5.80 x 10-9 g/L. If the value of gold is $370.00/troy ounce, what is the value of gold in the Earth's oceans with 1 troy ounce = 31.1 g. Group of answer choices $2.83 x1027 $2.83 x1033 $9.21 x109 $9.52 x1013 $9.52 x106 $9.21 x1016

