How many moles of O2 are needed to burn 1.50 molmol of C8H18? Express the amount in moles to three significant digits. How many grams of O2 are needed to burn 14.0 gg of C8H18? Express the mass in grams to three significant digits. Octane has a density of 0.692 g/mLat 20∘C. How many grams of O2 are required to burn 19.0 gal of C8H18? Express the mass in grams to three significant digits.
How many moles of O2 are needed to burn 1.50 molmol of C8H18? Express the amount in moles to three significant digits. How many grams of O2 are needed to burn 14.0 gg of C8H18? Express the mass in grams to three significant digits. Octane has a density of 0.692 g/mLat 20∘C. How many grams of O2 are required to burn 19.0 gal of C8H18? Express the mass in grams to three significant digits.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.23P
Related questions
Question
How many moles of O2 are needed to burn 1.50 molmol of C8H18?
Express the amount in moles to three significant digits.
How many grams of O2 are needed to burn 14.0 gg of C8H18?
Express the mass in grams to three significant digits.
Octane has a density of 0.692 g/mLat 20∘C. How many grams of O2 are required to burn 19.0 gal of C8H18?
Express the mass in grams to three significant digits.
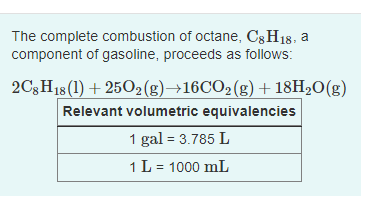
Transcribed Image Text:The complete combustion of octane, C3H18, a
component of gasoline, proceeds as follows:
2C3H18 (1) + 2502(g)→16CO2(g) + 18H2O(g)
Relevant volumetric equivalencies
1 gal = 3.785 L
1L = 1000 mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co