Q: I-ö: I 0-Ö: Z-I termine the molecular geometry arround each of the central atoms in the molecule…
A:
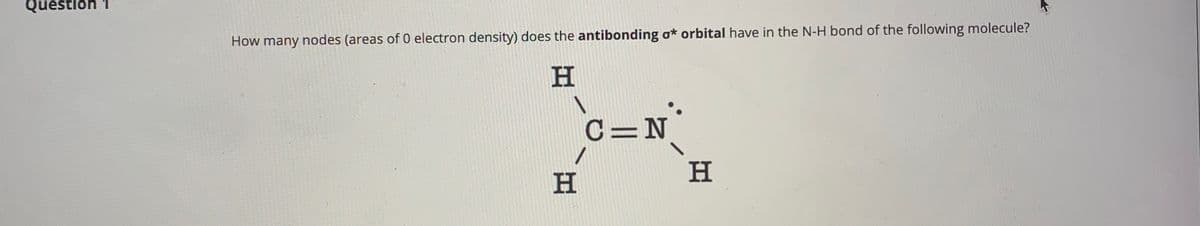
Q: N-H I II III
A: To solve this question, the hybridization of nitrogen and structural aspects such as delocalization…
Q: How many nodes does the highest نقطتان )2( energy molecular orbital of 1,3,5,7- octatetraene have 4…
A:
Q: Describe each highlighted bond in terms of the overlap of atomic orbitals
A: The number of sigma bonds give the information of hybridisation present in the molecule.
Q: Which of the species contains a delocalized x bond? H,O HCN O CO - co3-
A:
Q: Identify all of the carbon atoms that are sp2-hybridized in the following molecule: H :ci: :či: :0:…
A:
Q: Which compound has a larger dipole moment: CH3Cl or CH2Cl2?
A: To determine the compound with larger dipole moment
Q: 1. Which bond-line structure, as drawn, is identical to the given Newman projection? CH3 H. CH3 F а.…
A: OPTION- B IS CORRECT CHOICE ACCORDING TO ME AS BOTH CH3 GROUP IS ANTI TO EACH OTHER IN NEWMAN…
Q: The following two compounds are isomers; that is, they are different compounds with the same…
A:
Q: Which molecule has the largest overall dipole? A CI H;C CI CH3 CH3 C=C O=C CI CI CH3 CH3 CH3
A:
Q: Onto which of the numbered atoms in the structure can the negative charge be moved by resonance…
A: Answer:- This question is answered by using the simple concept of writing the resonating structures…
Q: Do the four carbons in the following compound always lie on the same plane?
A: One can predict the sterochemistry of any compound by looking at it 3-D structure. Many times…
Q: A 3D representation of a cyclohexane (C,H12) molecule, a cyclic compound used in the manufacture of…
A: First the atomic orbitals of carbon undergoes hybridization to form hybrid orbitals with 1S orbital…
Q: 44. In the molecular orbital model of cyclobutadiene, how many T-antibonding molecular orbitals are…
A:
Q: Describe each highlighted bond in terms of the overlap of atomic orbitals
A: Interpretation: The orbital overlapping taking place has to be described for the highlighted bond of…
Q: A € =E-C € = 6-C-e B
A: The process by which electrons are transferred between adjacent atoms through the bonds is called…
Q: What orbitals are used to form the labeled bonds in the following molecule? Of the labeled C—C…
A: 1--> C-H bond is formed due to overlap of sp2-s orbitals 2--> C-C bond is formed due to…
Q: Describe each highlighted bond in terms of the overlap of atomic orbitals
A:
Q: Which of the bonds shown in red in each compound or pair of compounds is shorter?
A: “Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for…
Q: What orbital do the lone-pair electrons occupy in each of the following compounds?
A: The Lewis structure does not give any idea about molecular geometry but it purposed the distribution…
Q: Given the following linear molecule, on which part of the molecule is the electron density the…
A:
Q: Identify all of the carbon atoms that are sp2-hybridized in the following molecule: :o: H :ci; H INE…
A: Single bonded carbon is sp3 hybridized Double bonded carbon is sp2 hybridized Triple bonded carbon…
Q: 4. What is the hybridization of either carbon atom in ethene? Which orbitals overlap to form the C-C…
A:
Q: H H -C-C H C-H H H d. H H е. NH2
A: The hybridization of different carbon atoms in the different molecules is as follows:
Q: H :0: H-N-C-C, 0-H A H What is the molecular geometry about the carbon labeled B in the molecule…
A: Trigonal planner due to sp2 hybridization
Q: There are two different systems of delocalized electrons in capilin, one containing more electrons…
A:
Q: Consider the structure of ascorbic acid below. НО 5 3 HO. O HỞ `H i. C5 is • hybridized. ii. How…
A: Consider the structure of ascorbic acid below.
Q: Describe each highlighted bond in terms of the overlap of atomic orbitals
A:
Q: In the molecule below, which orbitals make up the sigma bond between H and C₁? Which orbitals make…
A:
Q: How many unhybridized p atomic orbitals (add up the orbitals on all the atoms) are involved in pi…
A:
Q: A 3D representation of a cyclohexane (C,H12) molecule, a cyclic compound used in the manufacture of…
A:
Q: Which of the following describes the orbital overlap at bond B? Bond B *: *: А-А-А-X: :Y: :X::X:
A:
Q: How many electrons are in the bonding molecular orbital of Hen? 01 4. O O O O
A: Bonding molecular orbital:
Q: Streptimidone is an antibiotic whose structure is shown below. Specify the configurations around…
A: Given that a question of streptimidone structures. Difine a indicate of a and b carbon atom…
Q: What is the hybridization of the sigma bonding orbitals of the carbon atom?
A: The mixing of atomic orbitals which forms the hybridized orbitals of same characteristic and…
Q: The molecular geometry of the left-most carbon atom in the molecule below is H 0 нссон Н
A: we have to determine the molecular geometry
Q: H,N CH-C- CH C- CH3
A:
Q: a MO energy diagram with two electrons in sigma 2s two electrons in sigma 2s* and four electrons in…
A: According to MOT (molecular orbital theory ) 1. For 14 (or) below electrons molecular orbitals…
Q: An electron in N2 is excited from the highest occupied molecular orbital (HOMO) to the lowest…
A: As the electron excited from HOMO to LUMO, the bond order decreases from 3 to 2.
Q: H HH O. H-C-S-C-C C-C5 1² ]³ H. 2 3. 4 - 2. H. Which carbons have a tetrahedral molecular geometry…
A:
Q: a) Draw the structures of the following compounds showing every orbital involved in the sigma (o)…
A:
Q: Many reactions involve a change in hybridization of one or more atoms in the starting material. In…
A: When organic materials undergo chemical reaction they also undergo change in hybridization. This…
Q: H H H H H-Ć-Ć– C-N: H Molecular orbital type: エーO一エ エーO一エ エーO一エ
A: Sigma bonds are the strongest bonds in the chemical structures. The form by the head-on head…
Q: What orbitals are used to form the labeled bonds in the following molecule? Of the labeled C—C…
A: 1 number bond is C-H bond and in this bond carbon is sp2 hybridized and hydrogen has s orbital so…
Q: 1. Redraw the following molecule in the skeletal format. (CH3)2C=CHCH,CH(OH)CH,CH,CHO
A: According to the skeletal format,a bond between two atoms is shown by a line.
Q: Which of the following molecules is polar? O BeCl2 O CH2F2 O CH4 O AIF3
A: Given : We have to write which of the following is polar.
Q: Describe each highlighted bond in terms of the overlap of atomic orbitals
A:
Q: Which atomic orbitals overlap to form the C(1)-C(2) sigma bond in the following molecule? H H-C- EC…
A: Hybrid orbitals overlap and form the sigma bond. The hybrid orbitals that are overlapping can be…
Q: The molecular geometry of the left-most carbon atom in the molecule below is, H O H C-C0H A)…
A:
Explain the answer to this please :(
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

- The H2S bond angle has been experimentally measured to be close to 90 degree (not 109.5 degree). How can the 90 degree bond angle be better explained using hydrogen's 1st and sulfur's 3p atomic oribitals than with hybrid oribitals? I want a good simple answer, thanks.Answer all parts (A, B, & C) a) Draw the Lewis structure of the nitrate ion, NO3 -, including all resonance forms and formal charges. What is the geometry of the nitrate ion? b) True or False: Because of the double bond in the nitrate ion, two bond angles are greater than 120° and one bond angle is less than 120°. Explain your answer. c) What is the hybridization of the N in nitrate?10.12 Answer true or false. (a) In organic compounds, carbon normally has fourbonds and no unshared pairs of electrons. (b) When found in organic compounds, nitrogen nor-mally has three bonds and one unshared pair ofelectrons. (c) The most common bond angles about carbon inorganic compounds are approximately 109.5°and 180°.
- draw the 3d model of the molecule CH3CH2CH2C[triplebond]CCH2CH2CH3 from the view of the triple bonded C atoms. Not sure if I drew the appropriate hydrogens needed on the Carbons that have the triple bond?Which of the following compounds has bond angles of 120°? Explain why this is so. I still don't understand how to get the link angles, I hope you can help me.Select the correct value for the indicated bond angle in each of the compounds. O−S−O angle of SO2 O−S−O angle of SO3 F−O−F angle of OF2 Cl−Be−Cl angle of BeCl2 F−P−F angle of PF3 Cl−Si−Cl angle of SiCl4 Angle Option Answers for all listed above: 90° <109.5° 109.5° <120° 120° 180
- Select one of the following for the 1st problem- ☞Trigonal Planar ☞Trigonal Pyramidal ☞Linear ☞Bent ☞Tetrahedral 2)Given your geometry assignment of C2H4, which of the molecules investigated in this question has the same geometry for further investigation?Help! a.) Draw all the important resonance contributors for the structure shown below. Use curved arrows to show the movement of electrons between these contributors. b.) Draw the hybrid resonance hybrid structure.QUESTION 5 (ii) Show the direction of the dipole moment on the following compounds:a) CO2b) BF3c) H2Od) CH3Cl.
- Which of the following bonds is least polar? Select one: a. B-H b. Al-H c. P-H d. C-H e. N-HHello I am a bit confused and I need help. 1. Are inorganic compounds more susceptible compared to organic compounds when it comes to oxidation and combustion reaction? 2. Can carbon atoms bond to almost every element in the periodic table? 3. Do reactions between covalent organic molecules proceed more slowly and only happens at lower temperatures with or without catalysts? 4. In order to give four new hybrid orbitals, it is assumed that the four equivalent orbitals are formed by blending the 2p and the three 2s atomic orbitals of a carbon atom?Statement 1: The geometry formed from the bonding of C and H atoms for Compound 2 is trigonal Statement 2: Meaning, the distance between the two C atoms is closer than Compound 1 Which statement is true? 1, 2, both, or none?

