How many of the following molecules exhibit H-bonds, how many exhibit dipole-dipole interactions and how many exhibit London Dispersion forces respectively? H CH H CH H A. 1, 2 and 4 B. 2, 2 and 4 C. 2, 3 and 4 D. 2, 0 and 2 E. 1, 2 and 1 H-C-H CH3OH Н H₂S NH3 H. H H-C-O-H Н S. H H
How many of the following molecules exhibit H-bonds, how many exhibit dipole-dipole interactions and how many exhibit London Dispersion forces respectively? H CH H CH H A. 1, 2 and 4 B. 2, 2 and 4 C. 2, 3 and 4 D. 2, 0 and 2 E. 1, 2 and 1 H-C-H CH3OH Н H₂S NH3 H. H H-C-O-H Н S. H H
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter9: Liquids And Solids
Section: Chapter Questions
Problem 28QAP: Which of the following compounds would you expect to show dispersion forces? Dipole forces? (a)...
Related questions
Question
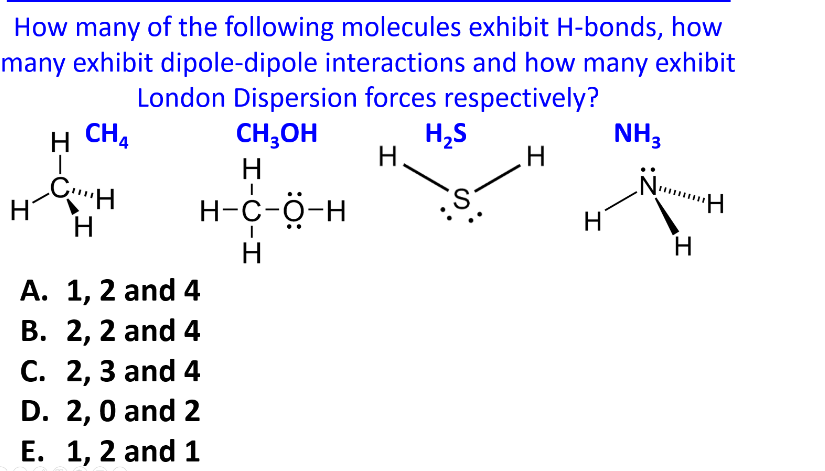
Transcribed Image Text:How many of the following molecules exhibit H-bonds, how
many exhibit dipole-dipole interactions and how many exhibit
London Dispersion forces respectively?
H CH
H
CH
H
A. 1, 2 and 4
B. 2, 2 and 4
C. 2, 3 and 4
D. 2, 0 and 2
E. 1, 2 and 1
H-C-H
CH3OH
Н
H₂S
NH3
H.
H
H-C-O-H
Н
S.
H
H
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning