Q: Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen…
A: Given data is as follows: ..... (1)…
Q: Let's suppose that K2CrO4 and Pb(ClO4)2 react following a double displacement reaction pattern. A.…
A: Ionic compound:Ionic compound composed of cation which is positively charged (+charge) and an anion…
Q: Oxygen gas can be generated by heating potassium chlorate: heat 2KCIO, (s) 2KCI(s) + 2KCI() + 30,…
A: We have to calculate the volume of oxygen gas produced.
Q: Draw a reasonable mechanism for the following transformation: For the mechanism, draw the curved…
A: The given reactant is a carbonyl compound and the reagents are sodium hydroxide, water and heat. The…
Q: Click the "draw structure" button to launch the drawing utility. Complete the following reaction by…
A: Base mediated ester hydrolysis also called saponification reaction gives alcohol and the carboxylate…
Q: What CO2 partial pressure (torr) is required to yield a velocity of 0.045 M-s1 for the reaction?…
A: The velocity is given as v = 0.045 M/sIt is required to find the pressure in torr.
Q: calculate the ph for each of the cases in the titration of 25.0 ml of .140 M pyrimidine with .140 M…
A: Since you have posted a question with multiple sub-parts, we will provide the solution only for the…
Q: Draw step 2 of the mechanism by completing the starting materials. wwww H₂C + NH; + Z +1 +1 +1 +1 1…
A: The question is based on Acid-Base reaction in the organic chemistry.
Q: Propose an appropriate base to carry out the transformation below and use it as part of a curly…
A: An arrow always depicts from a region of high electron density to low electron density ; that is…
Q: What stereoisomers are possible from the following reaction? Select all that apply. CI 0 0 O OH "OH…
A: The objective of the question is to predict the products formed from the given options for the…
Q: Consider the representation depicted in the molecular art for the reaction A + B C + D with an…
A: The given reaction is: So, the equilibrium constant of this reaction =
Q: 1.Os (excess) 2. Mežs ? MgBr equivalents) 2. H₂O Na Ory07 1. CH MgCl (2 equivalents) 2. HO (2…
A: The objective of the question is to predict the products formed in the following reaction given.
Q: er Write the mass balance for a K2CO3 solution if the species in the solution are K+, CO32-, HCO3,…
A: The species in the K2CO3 solution are:K+CO32-HCO3-H2CO3 H+OH-We need to calculate the mass balance…
Q: 4. Extraction: a) ( ) In what order (from lowest to highest R,) would you expect to find compound X,…
A: Thin layer chromatography (TLC) is a method of separating components of the mixture. It is based on…
Q: 2 c) Make models of the two possible isomers of the [Co(NH3)4C12]+ ion. This is an octahedral…
A: The objective of the question is to identify and name the two possible isomers of the [Co(NH3)4Cl2]+…
Q: Arrange the following substances according to their increasing acidity a) c) Fenol,…
A: The objective of the question is to arrange the given substances in the order of their increasing…
Q: CN ΗΝΟ; H2SO4
A: This is an example of nitration reaction
Q: (A)Write the Hückel Hamiltonian matrix for benzene. (B) The pictures below represent a top view of…
A: The objective of the question is to write the Huckel Hamiltonian matrix for benzene and to label the…
Q: Oxygen gas can be generated by heating potassium chlorate: heat 2KCIO, (s) 2KCI(s) + 302(g) What…
A:
Q: Draw a scheme showing the 4 possible stereoisomers of 2,3-dibromo-3-phenylpropanoic acid, indicating…
A: A pair of substances which are non-super imposable mirror images are called enantiomers.A pair of…
Q: Question 1. Indicate at which position(s) an electrophilic substitution will take place and justify…
A: When all the properties of a molecule cannot be explained by a single structure, we draw more than…
Q: What mass of precipitate (in g) is formed when 45.5 mL of 0.300 M K₃PO₄ reacts with 54.5 mL of 0.200…
A: The mass of CrPO4 precipitate formed is approximately 1.602 g.Explanation:The balanced chemical…
Q: For the reaction H2(g) + Br2(g) <-> 2HBr(g), Kc = 81.4 at 385C. What is the value of…
A: The value of equilibrium constant for the reaction is 0.012285.Explanation:Given, for the reaction…
Q: what do the equations in the third column have in common
A:
Q: Consider a buffer made by adding 132.8 g of NaC,H₂O₂ to 300.0 mL of 1.41 M HC,HO2 (Ka = 6.3 x 105) 7…
A: Answer:Buffer solution is a type of solution that resists the change in its pH on adding small…
Q: How many moles are in 78.1 grams of RbF?
A: The objective of this question is to calculate the number of moles in a given mass of a substance.…
Q: Question Aqueous hydrobromic acid HBr reacts with solid sodium hydroxide NaOH to produce…
A: Maas of HBr = 2.4 gMass of NaOH = 1.6 gMass of H2O produced = 0.155 g
Q: For the reaction 5Ce4+ + Mn 2+ + 4H2 O → 5Ce3+ + MnO4- + 8H+ , given E0 (Ce4+ /Ce3+ ) = 1.70V,E0…
A: ΔGo = 93108 J or 93.108 kJExplanation:The change in Gibbs free energy is the maximum amount of work…
Q: Compounds A, B, and C react according to the following equation. 3A(g) + 2B(g) <-> 2C(g) At…
A: Kc = 12.6Explanation:Given: [A]=0.536M;[B]=0.763M;[C]=1.063M3A(g)+2B(g)⇌2C(g)Kc=???Step 1: Write…
Q: 00 ? A B C N "INI* - 00 'N 00
A: Py is an abbreviation for pyridine
Q: Provide a curly arrow mechanism for this transformation, which is from a total synthesis of the…
A: This is an example of ketal formation. In a ketal formation reaction a ketone in the presence of…
Q: Draw both resonance structures of the most stable carbocation intermediate in the reaction shown. +…
A: In presence of HCl, H+ ion is generated which is accepted by electrophilic alkene centre and…
Q: Calculate the pH for each of the cases in the titration of 25.0 mL. of 0.220 M pyridine, CH,N(aq)…
A:
Q: Complete the following retrosynthesis. A 1 B 2 C 3 ☑ 4 L OH 1 OH HO ? 2 OH 3 HO Your answer میں 4 +…
A: It involves starting with a target compound and working backwards to break it down into simpler…
Q: What is the pH of a buffer that is 120 M in formic acid, HCHO2 and 0.080 M in potassium formate…
A: The objective of this question is to calculate the pH of a buffer solution. The buffer solution is…
Q: Name the following compounds and draw their structures: (5) a) [Fe(OH)( H2O)5] Cl2 b) Li[Cr(OH)4] c)…
A: The objective of the question is to name the given compounds and draw their structures. The…
Q: Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product…
A: In an organic reaction mechanism, a curved arrow represents the transfer of electrons. In an organic…
Q: [18] Which of following compound will undergo solvolysis with methanol to yield the two shown? H3C…
A:
Q: What is the pH of 0.055 M Sr(OH)2? 13.04 12.13 13.39 13.75 12.87
A: 13.04Explanation:Detailed explanation:Step 1: Determine the dissociation of Sr(OH)2. Being a…
Q: Draw the aromatic compounds named below. o-bromophenol Draw Your Solution
A:
Q: What are the electronic effects of the ketone on the C=C double bond? O O-1, +M O-I, -M O +1, +M O…
A: The question is asking about the electronic effects of a ketone on a carbon-carbon double bond. In…
Q: HO .OH cat. H2SO4 H₂O cat. H2SO4 Complete both mechanisms above. BRIEFLY explain how the different…
A: The objective of the question is to understand the mechanisms of two reactions catalyzed by H2SO4…
Q: Draw the major product of this reaction. Ignore inorganic byproducts. + NaOCH2CH3, CH3CH2OH Select…
A: The given reaction is based on Michael -addition reaction.The Michael addition reaction, also known…
Q: Don't use hand raiting please
A: The objective of this question is to calculate the pH of a solution that is 0.50 M in CH3NH3Cl.…
Q: Which of the compounds is more soluble in an acidic solution than in pure water? Be(OH)2 CuCN…
A:
Q: What reagent required for the following tra HOA HO 2.N но HROO LBH, THE 2.HO₂ NaOH 10 ? но
A: Find out suitable reagent for organic conversion
Q: Draw the structure for an alkene that gives the following reaction product. ? CH2l2, Zn/Cu • Ignore…
A: Zinc copper couple reacts with the diiodomethane to form organozinc carbenoid (similar to carbene).…
Q: Determine the mean paracetamol content per tablet (in mg), using the following values: the mean…
A: A=ϵ.c.lWhere:A is the absorbanceε is the molar absorption coefficient (in M−1⋅Cm−1)c is the…
Q: How many cis/trans isomers does this molecule have? Enter the number in the box above the drawing…
A:
Q: What is the highest-energy intermediate in the reaction profile below? Transition states OE OF OD O…
A: The state corresponding to highest potential energy.
Step by step
Solved in 3 steps with 2 images

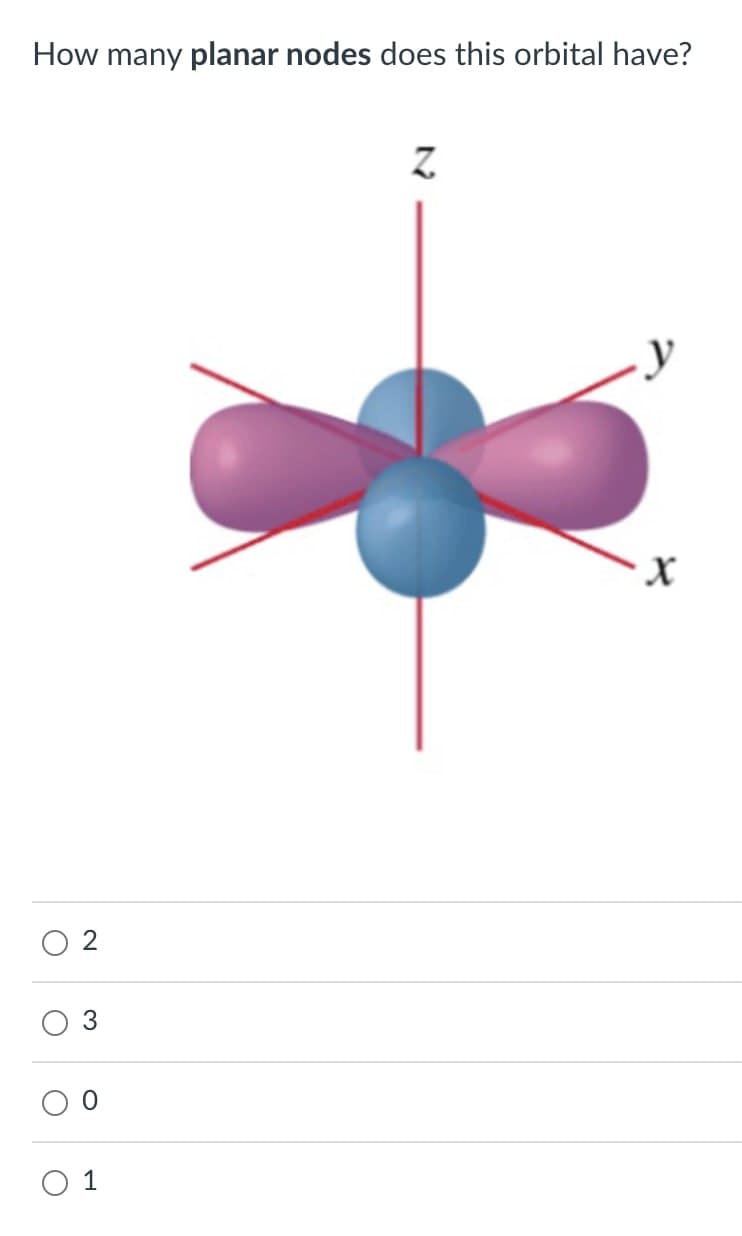
- show a 4dz2 orbitalWhy is there electron subshell overlap in the 4s and 3d orbitals?Draw sketches illustrating the overlap between the followingorbitals on two atoms: (a) the 2s orbital on each atom,(b) the 2pz orbital on each atom (assume both atoms are onthe z-axis), (c) the 2s orbital on one atom and the 2pz orbitalon the other atom.
- True or false? The 4d orbital does not exist in the carbon atom. Justify your answer in 1 sentence or 2.Which of the following represents the electron configuration of S⁻? A) 1s²2s²2p⁶3s²3p⁶ B) 1s²2s²2p⁶3s²3p⁵ C) 1s²2s²2p⁶3s²3p⁴ D) 1s²2s²2p⁶3s²3p³ E) 1s²2s²2p⁶3s²3p²T/F: A p orbital on one atom cannot overlap an s orbital on another atom.
- Which of the following has about 109.5° bond angle? Select one: a.SF4 b.IF4– c.SiF4 d.XeF4 Following the convention of spin-up before spin-down electrons, how many electrons are there in a d-orbital having the last entering electron with a mℓ = 0, and ms = –1/2? Select one: a.8 b.10 c.3 d.5Which of the following represents the electron configuration of P³⁻? A) 1s²2s²2p⁶3s²3p⁶ B) 1s²2s²2p⁶3s²3p³ C) 1s²2s²2p⁶3s² D) 1s²2s²2p⁶3s²3p⁶4s¹ E) 1s²2s²2p⁶3s²3p¹ii) What are the differences between a 4py orbital and a 3dx2-y2 orbital in the H atom?

