Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 19E: Give the number of protons, electrons, and neutrons in neutral atoms of each of the following...
Related questions
Question
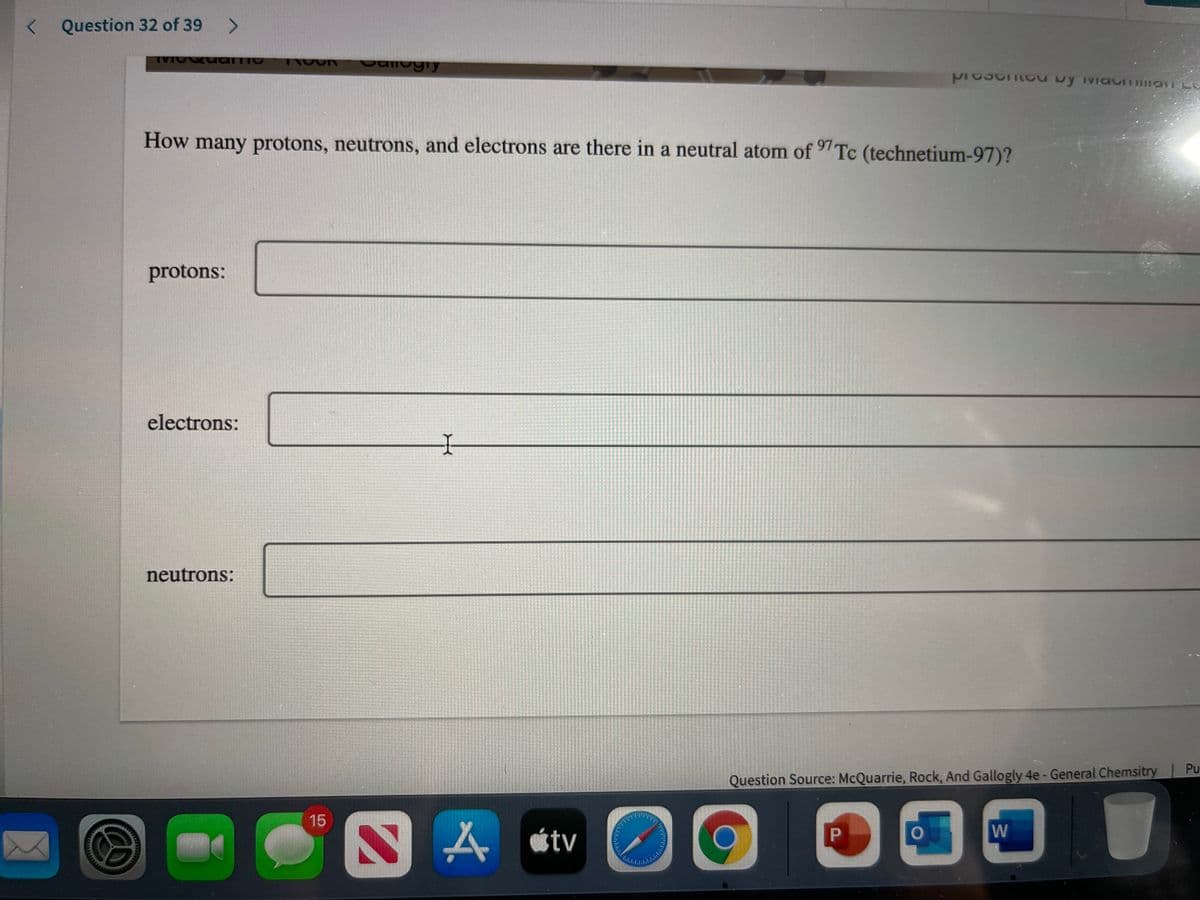
Transcribed Image Text:Question 32 of 39 >
How many protons, neutrons, and electrons are there in a neutral atom of Tc (technetium-97)?
protons:
electrons:
neutrons:
Pu
Question Source: McQuarrie, Rock, And Gallogly 4e- General Chemsitry
15
人tv
שיהה 4
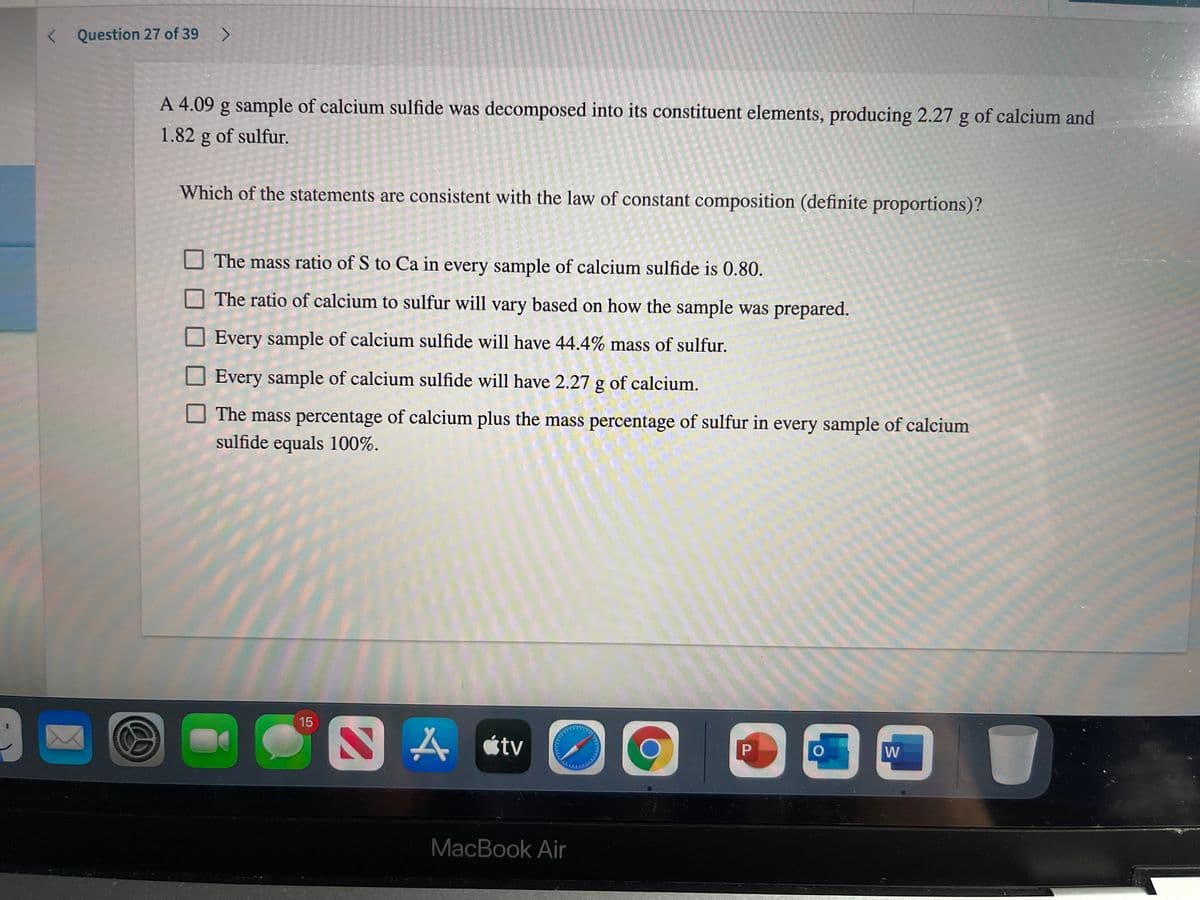
Transcribed Image Text:< Question 27 of 39 >
A 4.09 g sample of calcium sulfide was decomposed into its constituent elements, producing 2.27 g of calcium and
1.82 g of sulfur.
Which of the statements are consistent with the law of constant composition (definite proportions)?
O The mass ratio of S to Ca in every sample of calcium sulfide is 0.80.
The ratio of calcium to sulfur will vary based on how the sample was prepared.
Every sample of calcium sulfide will have 44.4% mass of sulfur.
O Every sample of calcium sulfide will have 2.27 g of calcium.
The mass percentage of calcium plus the mass percentage of sulfur in every sample of calcium
sulfide equals 100%.
15
A étv
W
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning