How much energy is required to change a 60-g ice cube from ice at -40°C to steam at 130°C? Enter a number. differs from the correct answer by more than 10%. Double check your calculations. M) Read It Need Help?
How much energy is required to change a 60-g ice cube from ice at -40°C to steam at 130°C? Enter a number. differs from the correct answer by more than 10%. Double check your calculations. M) Read It Need Help?
Physics for Scientists and Engineers: Foundations and Connections
1st Edition
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Katz, Debora M.
Chapter21: Heat And The First Law Of Thermodynamics
Section: Chapter Questions
Problem 4PQ
Related questions
Question
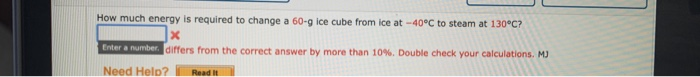
Transcribed Image Text:How much energy is required to change a 60-g ice cube from ice at -40°C to steam at 130°C?
Enter a number. differs from the correct answer by more than 10%. Double check your calculations. M)
Read It
Need Help?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning
