2.18 Perform a degrees of freedom analysis for the model in Eqs. 2-64 through 2-68. Identify parameters, output variables, and inputs (manipulated and disturbance variables). tions in the inlet Mixture of A and B mics of the pro- omatic control is 9, CA, T V, p, T 2.4 Dyna reaction. In other words, the heat of mixing is neg- ligible compared to the heat of reaction. 8. Shaft work and heat losses to the ambient can be neglected. decrease in lelllllld displayed in centration as The following form of the CSTR energy balance is convenient for analysis and can be derived from Eqs. 2-62 and 2-63 and Assumptions 1-8 (Fogler, 2006; Russell and Denn, 1972), TR) have wide- embody many STR models tend types of continu- and packed-bed odel provides a ing principles for Table 2.3 Nc Cooling medium at temperature T. Parameter VpcdT = wC(T; - T) + (-AHR)Vkc, Figure 2.6 A nonisothermal continuous stirred-tank reactor. dt + UA(T. – T) (2-68) For these assumptions, the unsteady-state mass balance { for the CSTR is where AHR is the heat of reaction per mole of A that is reacted. In summary, the dynamic model of the CSTR consists of Eqs. 2-62 to 2-64, 2-66, 2-67, and 2-68. This model is nonlinear as a result of the many product terms and Tne exponential temperature dependence of k in Eq. 2-63. Consequently, it must be solved by numerical integra- tion techniques (Fogler, 2006). The CSTR model will become considerably more complex if -AHR versible chemical ets to form species B. We assume that d(pV) (2-64) = pq, - pg dt Because V and p are constant, Eq. 2-64 reduces to 450 respect to compo- (2-65) q = 9i Thus, even though the inlet and outlet flow rates may change due to upstream or downstream conditions, Eq. 2-65 must be satisfied at all times. In Fig. 2.6, both flow rates are denoted by the symbol q. For the stated assumptions, the unsteady-state com- ponent balances for species A (in molar concentration units) is (2-62) e 400- 1. More complicated rate expressions are considered. For example, a mass action kinetics model for a second-order, irreversible reaction, 2A B, is given by per unit volume, units of reciprocal tion of species A. onstant is typically ature given by the 1 = kzc 350 ---. (2-69) 2. Additional species or chemical reactions are involved. If the reaction mechanism involved pro- duction of an intermediate species, 2A B → B, then unsteady-state component balances for both A and B* would be necessary (to calculate c, and CR), or balances for both A and B could be written (to calculate c, and cB). Information concerning the reaction mechanisms would also be required. y dea = q(CAi - CA) – VkcA (2-66) 300 dt (2-63) This balance is a special case of the general component balance in Eq. 2-7. Next, balance for the CSTR. But first we make five additional Figure 2.7 Rea. changes in cool and from 300 to E is the activation The expressions in theoretical consid- and E are usually consider an unsteady-state energy we Reactions involving multiple species are described by high-order, highly coupled, nonlinear reaction models, because several component balances must be written. assumptions: 1.0 4. The thermal capacitances of the coolant and the cooling coil wall are negligible compared to the thermal capacitance of the liquid in the tank. 5. All of the coolant is at a uniform temperature, T- (That is, the increase in coolant temperature as the coolant passes through the coil is neglected.) 0.9 data. Thus, these obe semi-empirical in Section 2.2. CSTR is shown in 0.8 EXAMPLE 2.5 0.7 To illustrate how the CSTR can exhibit nonlinear dynamic behavior, we simulate the effect of a step change in the coolant temperature T, in positive and negative directions. Table 2.3 shows the parameters and nominal operating condition for the CSTR based on Eqs. 2-66 and 2-68 for the exothermic, irreversible first-order reaction A two state variables of the ODES are the concentration of A (c.) and the reactor temperature T. The manipulated input variable is the jacket water temperature, T Two cases are simulated, one based on increased cool- ing by changing T, from 300 to 290 K and one reducing the cooling rate by increasing T, from 300 to 305 K. These model equations are solved in MATLAB with a numerical integrator (ode15s) over a 10-min horizon. The 0.6 of pure component cooling coil is used t the desired oper- cat that is released 0.5 0.4 6. The rate of heat transfer from the reactor contents to the coolant is given by 0.3 0.2- 0.1 B. The nitial CSTR model (2-67) Q = UA(T- T) mptions: where U is the overall heat transfer coefficient and A is the heat transfer area. Both of these model parameters are assumed to be constant. 7. The enthalpy change associated with the mixing of the feed and the liquid in the tank is negligible com- pared with the enthalpy change for the chemical 0. Figure 2.8 React. changes in coolin and product streams are denoted by p. ctor is kept constant Reactant A concentration (mol/L) Reactor temperature (K):
2.18 Perform a degrees of freedom analysis for the model in Eqs. 2-64 through 2-68. Identify parameters, output variables, and inputs (manipulated and disturbance variables). tions in the inlet Mixture of A and B mics of the pro- omatic control is 9, CA, T V, p, T 2.4 Dyna reaction. In other words, the heat of mixing is neg- ligible compared to the heat of reaction. 8. Shaft work and heat losses to the ambient can be neglected. decrease in lelllllld displayed in centration as The following form of the CSTR energy balance is convenient for analysis and can be derived from Eqs. 2-62 and 2-63 and Assumptions 1-8 (Fogler, 2006; Russell and Denn, 1972), TR) have wide- embody many STR models tend types of continu- and packed-bed odel provides a ing principles for Table 2.3 Nc Cooling medium at temperature T. Parameter VpcdT = wC(T; - T) + (-AHR)Vkc, Figure 2.6 A nonisothermal continuous stirred-tank reactor. dt + UA(T. – T) (2-68) For these assumptions, the unsteady-state mass balance { for the CSTR is where AHR is the heat of reaction per mole of A that is reacted. In summary, the dynamic model of the CSTR consists of Eqs. 2-62 to 2-64, 2-66, 2-67, and 2-68. This model is nonlinear as a result of the many product terms and Tne exponential temperature dependence of k in Eq. 2-63. Consequently, it must be solved by numerical integra- tion techniques (Fogler, 2006). The CSTR model will become considerably more complex if -AHR versible chemical ets to form species B. We assume that d(pV) (2-64) = pq, - pg dt Because V and p are constant, Eq. 2-64 reduces to 450 respect to compo- (2-65) q = 9i Thus, even though the inlet and outlet flow rates may change due to upstream or downstream conditions, Eq. 2-65 must be satisfied at all times. In Fig. 2.6, both flow rates are denoted by the symbol q. For the stated assumptions, the unsteady-state com- ponent balances for species A (in molar concentration units) is (2-62) e 400- 1. More complicated rate expressions are considered. For example, a mass action kinetics model for a second-order, irreversible reaction, 2A B, is given by per unit volume, units of reciprocal tion of species A. onstant is typically ature given by the 1 = kzc 350 ---. (2-69) 2. Additional species or chemical reactions are involved. If the reaction mechanism involved pro- duction of an intermediate species, 2A B → B, then unsteady-state component balances for both A and B* would be necessary (to calculate c, and CR), or balances for both A and B could be written (to calculate c, and cB). Information concerning the reaction mechanisms would also be required. y dea = q(CAi - CA) – VkcA (2-66) 300 dt (2-63) This balance is a special case of the general component balance in Eq. 2-7. Next, balance for the CSTR. But first we make five additional Figure 2.7 Rea. changes in cool and from 300 to E is the activation The expressions in theoretical consid- and E are usually consider an unsteady-state energy we Reactions involving multiple species are described by high-order, highly coupled, nonlinear reaction models, because several component balances must be written. assumptions: 1.0 4. The thermal capacitances of the coolant and the cooling coil wall are negligible compared to the thermal capacitance of the liquid in the tank. 5. All of the coolant is at a uniform temperature, T- (That is, the increase in coolant temperature as the coolant passes through the coil is neglected.) 0.9 data. Thus, these obe semi-empirical in Section 2.2. CSTR is shown in 0.8 EXAMPLE 2.5 0.7 To illustrate how the CSTR can exhibit nonlinear dynamic behavior, we simulate the effect of a step change in the coolant temperature T, in positive and negative directions. Table 2.3 shows the parameters and nominal operating condition for the CSTR based on Eqs. 2-66 and 2-68 for the exothermic, irreversible first-order reaction A two state variables of the ODES are the concentration of A (c.) and the reactor temperature T. The manipulated input variable is the jacket water temperature, T Two cases are simulated, one based on increased cool- ing by changing T, from 300 to 290 K and one reducing the cooling rate by increasing T, from 300 to 305 K. These model equations are solved in MATLAB with a numerical integrator (ode15s) over a 10-min horizon. The 0.6 of pure component cooling coil is used t the desired oper- cat that is released 0.5 0.4 6. The rate of heat transfer from the reactor contents to the coolant is given by 0.3 0.2- 0.1 B. The nitial CSTR model (2-67) Q = UA(T- T) mptions: where U is the overall heat transfer coefficient and A is the heat transfer area. Both of these model parameters are assumed to be constant. 7. The enthalpy change associated with the mixing of the feed and the liquid in the tank is negligible com- pared with the enthalpy change for the chemical 0. Figure 2.8 React. changes in coolin and product streams are denoted by p. ctor is kept constant Reactant A concentration (mol/L) Reactor temperature (K):
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%

Transcribed Image Text:2.18 Perform a degrees of freedom analysis for the model in
Eqs. 2-64 through 2-68. Identify parameters, output variables,
and inputs (manipulated and disturbance variables).
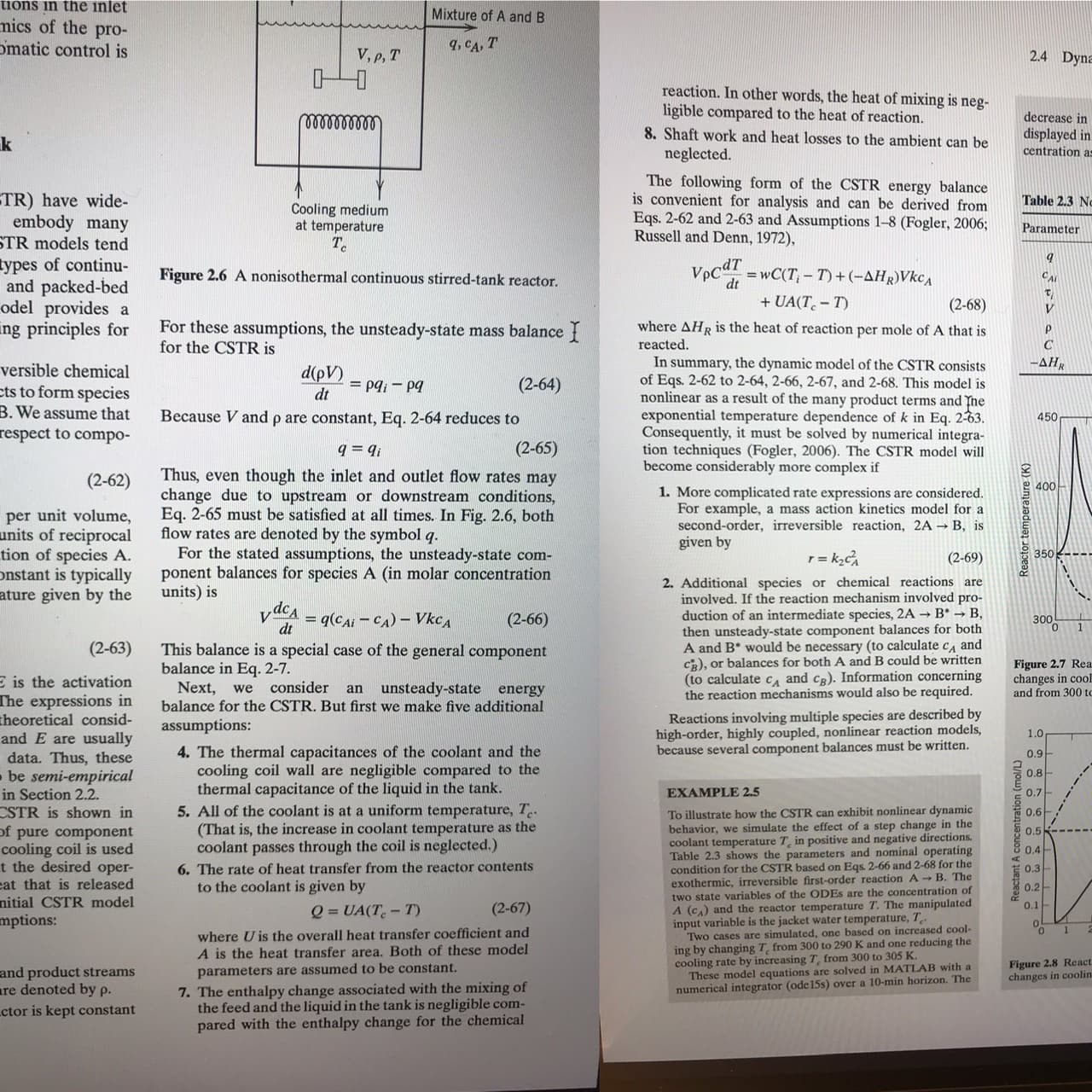
Transcribed Image Text:tions in the inlet
Mixture of A and B
mics of the pro-
omatic control is
9, CA, T
V, p, T
2.4 Dyna
reaction. In other words, the heat of mixing is neg-
ligible compared to the heat of reaction.
8. Shaft work and heat losses to the ambient can be
neglected.
decrease in
lelllllld
displayed in
centration as
The following form of the CSTR energy balance
is convenient for analysis and can be derived from
Eqs. 2-62 and 2-63 and Assumptions 1-8 (Fogler, 2006;
Russell and Denn, 1972),
TR) have wide-
embody many
STR models tend
types of continu-
and packed-bed
odel provides a
ing principles for
Table 2.3 Nc
Cooling medium
at temperature
T.
Parameter
VpcdT
= wC(T; - T) + (-AHR)Vkc,
Figure 2.6 A nonisothermal continuous stirred-tank reactor.
dt
+ UA(T. – T)
(2-68)
For these assumptions, the unsteady-state mass balance {
for the CSTR is
where AHR is the heat of reaction per mole of A that is
reacted.
In summary, the dynamic model of the CSTR consists
of Eqs. 2-62 to 2-64, 2-66, 2-67, and 2-68. This model is
nonlinear as a result of the many product terms and Tne
exponential temperature dependence of k in Eq. 2-63.
Consequently, it must be solved by numerical integra-
tion techniques (Fogler, 2006). The CSTR model will
become considerably more complex if
-AHR
versible chemical
ets to form species
B. We assume that
d(pV)
(2-64)
= pq, - pg
dt
Because V and p are constant, Eq. 2-64 reduces to
450
respect to compo-
(2-65)
q = 9i
Thus, even though the inlet and outlet flow rates may
change due to upstream or downstream conditions,
Eq. 2-65 must be satisfied at all times. In Fig. 2.6, both
flow rates are denoted by the symbol q.
For the stated assumptions, the unsteady-state com-
ponent balances for species A (in molar concentration
units) is
(2-62)
e 400-
1. More complicated rate expressions are considered.
For example, a mass action kinetics model for a
second-order, irreversible reaction, 2A B, is
given by
per unit volume,
units of reciprocal
tion of species A.
onstant is typically
ature given by the
1 = kzc
350 ---.
(2-69)
2. Additional species or chemical reactions are
involved. If the reaction mechanism involved pro-
duction of an intermediate species, 2A B → B,
then unsteady-state component balances for both
A and B* would be necessary (to calculate c, and
CR), or balances for both A and B could be written
(to calculate c, and cB). Information concerning
the reaction mechanisms would also be required.
y dea
= q(CAi - CA) – VkcA
(2-66)
300
dt
(2-63)
This balance is a special case of the general component
balance in Eq. 2-7.
Next,
balance for the CSTR. But first we make five additional
Figure 2.7 Rea.
changes in cool
and from 300 to
E is the activation
The expressions in
theoretical consid-
and E are usually
consider
an unsteady-state energy
we
Reactions involving multiple species are described by
high-order, highly coupled, nonlinear reaction models,
because several component balances must be written.
assumptions:
1.0
4. The thermal capacitances of the coolant and the
cooling coil wall are negligible compared to the
thermal capacitance of the liquid in the tank.
5. All of the coolant is at a uniform temperature, T-
(That is, the increase in coolant temperature as the
coolant passes through the coil is neglected.)
0.9
data. Thus, these
obe semi-empirical
in Section 2.2.
CSTR is shown in
0.8
EXAMPLE 2.5
0.7
To illustrate how the CSTR can exhibit nonlinear dynamic
behavior, we simulate the effect of a step change in the
coolant temperature T, in positive and negative directions.
Table 2.3 shows the parameters and nominal operating
condition for the CSTR based on Eqs. 2-66 and 2-68 for the
exothermic, irreversible first-order reaction A
two state variables of the ODES are the concentration of
A (c.) and the reactor temperature T. The manipulated
input variable is the jacket water temperature, T
Two cases are simulated, one based on increased cool-
ing by changing T, from 300 to 290 K and one reducing the
cooling rate by increasing T, from 300 to 305 K.
These model equations are solved in MATLAB with a
numerical integrator (ode15s) over a 10-min horizon. The
0.6
of pure component
cooling coil is used
t the desired oper-
cat that is released
0.5
0.4
6. The rate of heat transfer from the reactor contents
to the coolant is given by
0.3
0.2-
0.1
B. The
nitial CSTR model
(2-67)
Q = UA(T- T)
mptions:
where U is the overall heat transfer coefficient and
A is the heat transfer area. Both of these model
parameters are assumed to be constant.
7. The enthalpy change associated with the mixing of
the feed and the liquid in the tank is negligible com-
pared with the enthalpy change for the chemical
0.
Figure 2.8 React.
changes in coolin
and product streams
are denoted by p.
ctor is kept constant
Reactant A concentration (mol/L)
Reactor temperature (K):
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 12 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The