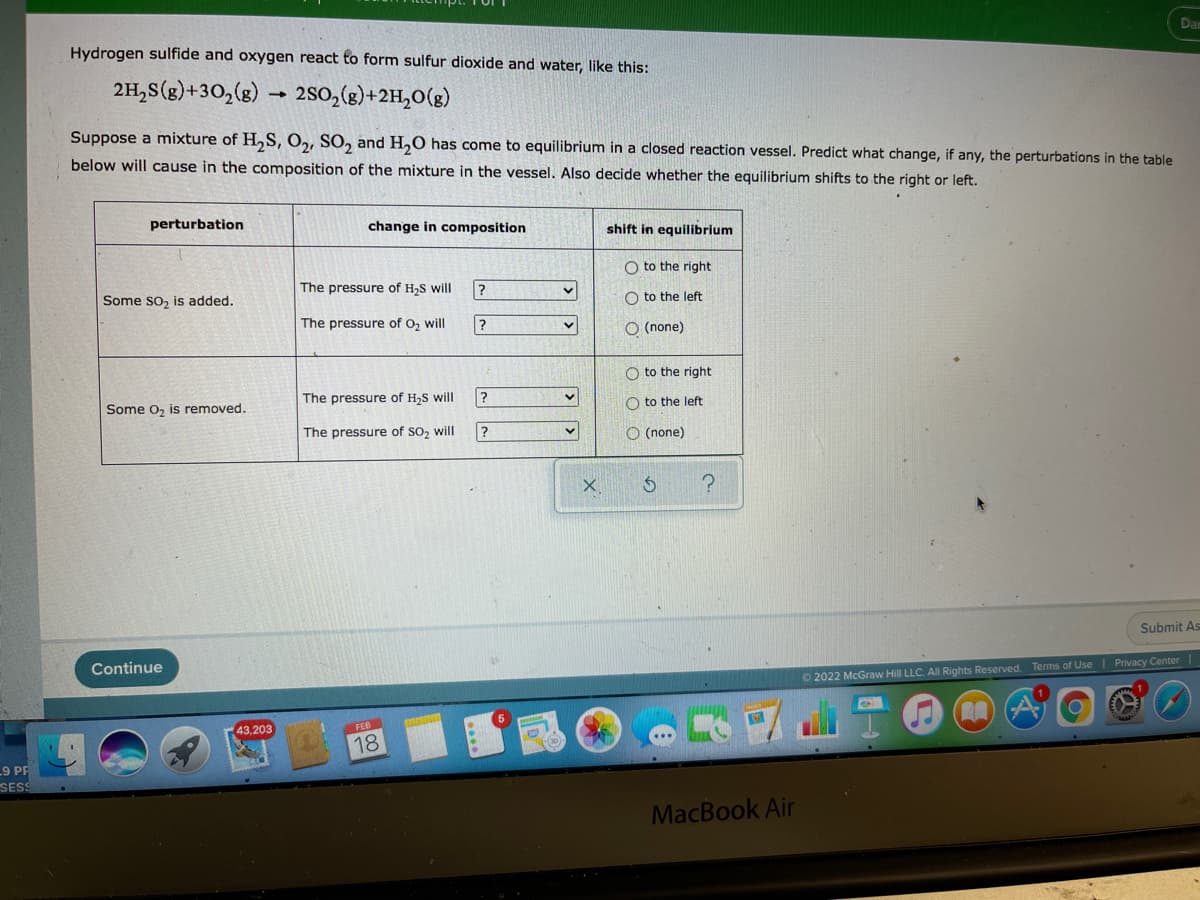
Hydrogen sulfide and oxygen react to form sulfur dioxide and water, like this: 2H,s(g)+30,(g) → 2S0,(g)+2H,O(g) Suppose a mixture of H,S, O,, SO, and H,O has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. perturbation change in composition shift in equilibrium O to the right The pressure of H,S will Some so, is added. O to the left The pressure of O, will O (none) O to the right The pressure of H,S will O to the left Some o, is removed. The pressure of SO, will ? O (none)
Hydrogen sulfide and oxygen react to form sulfur dioxide and water, like this: 2H,s(g)+30,(g) → 2S0,(g)+2H,O(g) Suppose a mixture of H,S, O,, SO, and H,O has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. perturbation change in composition shift in equilibrium O to the right The pressure of H,S will Some so, is added. O to the left The pressure of O, will O (none) O to the right The pressure of H,S will O to the left Some o, is removed. The pressure of SO, will ? O (none)
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter17: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 121CP: If wet silver carbonate is dried in a stream of hot air. the air must have a certain concentration...
Related questions
Question
Transcribed Image Text:Da
Hydrogen sulfide and oxygen react to form sulfur dioxide and water, like this:
2H,S(g)+30,(8) – 2s0,(g)+2H,0(g)
Suppose a mixture of H,S, O,, SO, and H,0 has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table
below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left.
perturbation
change in composition
shift in equilibrium
O to the right
Some so, is added.
The pressure of H2S will
O to the left
The pressure of O, will
O (none)
O to the right
Some o, is removed.
The pressure of H,S will
O to the left
The pressure of SO, will
O (none)
Submit As
Continue
O2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center
43,203
FEB
18
9 PP
SESS
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning