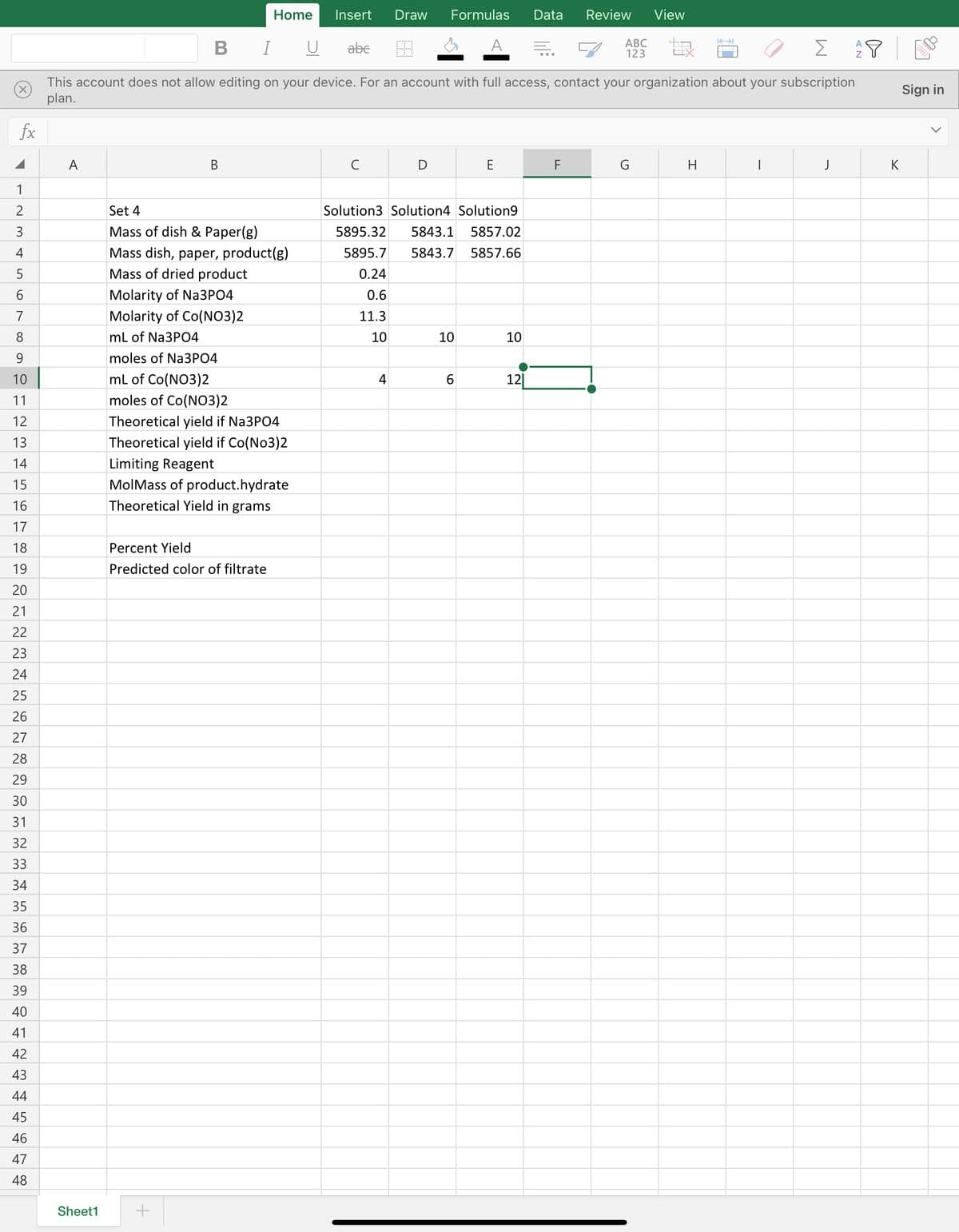
I am having trouble finding the rest of the data for my precipitation Lab for percent yield of cobalt phosphate. The data I collected has already been imputed but cannot find the rest of the data. I need to use the given data I collected to find the other blank boxes in excel.
I am having trouble finding the rest of the data for my precipitation Lab for percent yield of cobalt phosphate. The data I collected has already been imputed but cannot find the rest of the data. I need to use the given data I collected to find the other blank boxes in excel.
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 109AE: Patients undergoing an upper gastrointestinal tract laboratory test are typically given an X-ray...
Related questions
Question
I am having trouble finding the rest of the data for my precipitation Lab for percent yield of cobalt phosphate. The data I collected has already been imputed but cannot find the rest of the data. I need to use the given data I collected to find the other blank boxes in excel.
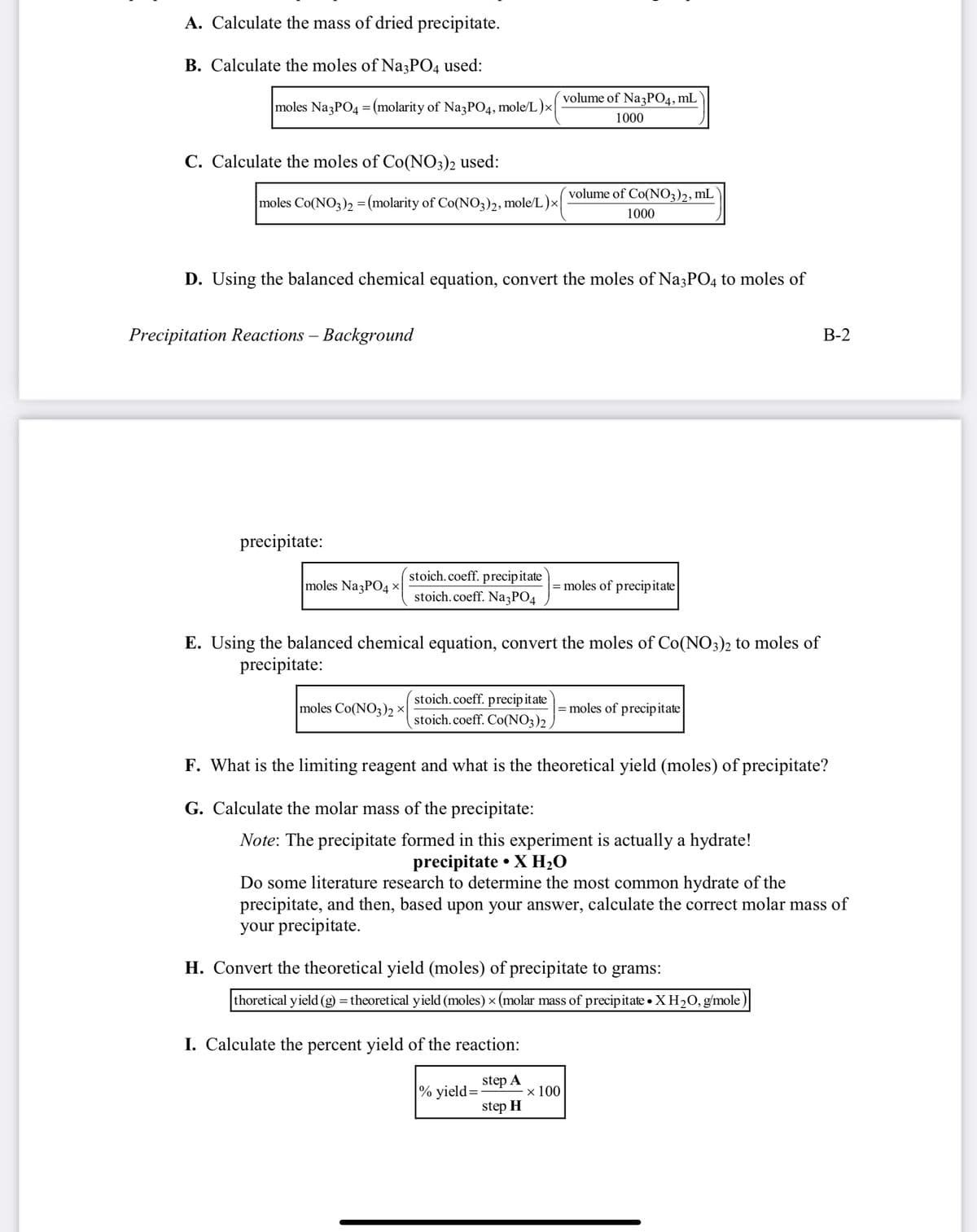
Transcribed Image Text:A. Calculate the mass of dried precipitate.
B. Calculate the moles of Na3PO4 used:
volume of NazPO4, mL
|moles Na3PO4 = (molarity of Na3PO4, mole/L )×
1000
C. Calculate the moles of Co(NO3)2 used:
volume of Co(NO3)2, mL
moles Co(NO3)2 = (molarity of Co(NO;)2, mole/L)×|
1000
D. Using the balanced chemical equation, convert the moles of Na3PO4 to moles of
Precipitation Reactions – Background
В-2
precipitate:
stoich.coeff. precipitate
stoich.coeff. Na3PO4
moles Na3PO4 x
moles of precipitate
E. Using the balanced chemical equation, convert the moles of Co(NO3)2 to moles of
precipitate:
stoich.coeff. precip itate
moles Co(NO3)2 ×
= moles of precipitate
stoich.coeff. Co(NO3)2
F. What is the limiting reagent and what is the theoretical yield (moles) of precipitate?
G. Calculate the molar mass of the precipitate:
Note: The precipitate formed in this experiment is actually a hydrate!
precipitate • X H2O
Do some literature research to determine the most common hydrate of the
precipitate, and then, based upon your answer, calculate the correct molar mass of
your precipitate.
H. Convert the theoretical yield (moles) of precipitate to grams:
thoretical yield (g) = theoretical yield (moles) × (molar mass of precipitate • X H20, g/mole)|
I. Calculate the percent yield of the reaction:
step A
|% yield=:
x 100
step H
Transcribed Image Text:Home
Insert
Draw
Formulas
Data
Review
View
B I
U
abe
A
АВС
123
Σ
This account does not allow editing on your device. For an account with full access, contact your organization about your subscription
plan.
Sign in
fx
A
В
C
E
F
H
J
K
1
2
Set 4
Solution3 Solution4 Solution9
3
Mass of dish & Paper(g)
5895.32
5843.1
5857.02
Mass dish, paper, product(g)
Mass of dried product
4
5895.7
5843.7
5857.66
5
0.24
6
Molarity of Na3PO4
0.6
7
Molarity of Co(NO3)2
11.3
8
mL of Na3PO4
10
10
10
9
moles of Na3PO4
10
mL of Co(NO3)2
4
6.
12|
11
moles of Co(NO3)2
12
Theoretical yield if Na3PO4
13
Theoretical yield if Co(No3)2
14
Limiting Reagent
15
MolMass of product.hydrate
16
Theoretical Yield in grams
17
18
Percent Yield
19
Predicted color of filtrate
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
Sheet1
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 9 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

