Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter23: Amines
Section: Chapter Questions
Problem 23.68P: Show how the synthetic scheme developed in Problem 23.67 can be modified to synthesize this...
Related questions
Question
I need help explaing this mechanism
Diels-Alder Reaction between anthracene and Maleic Anhydride
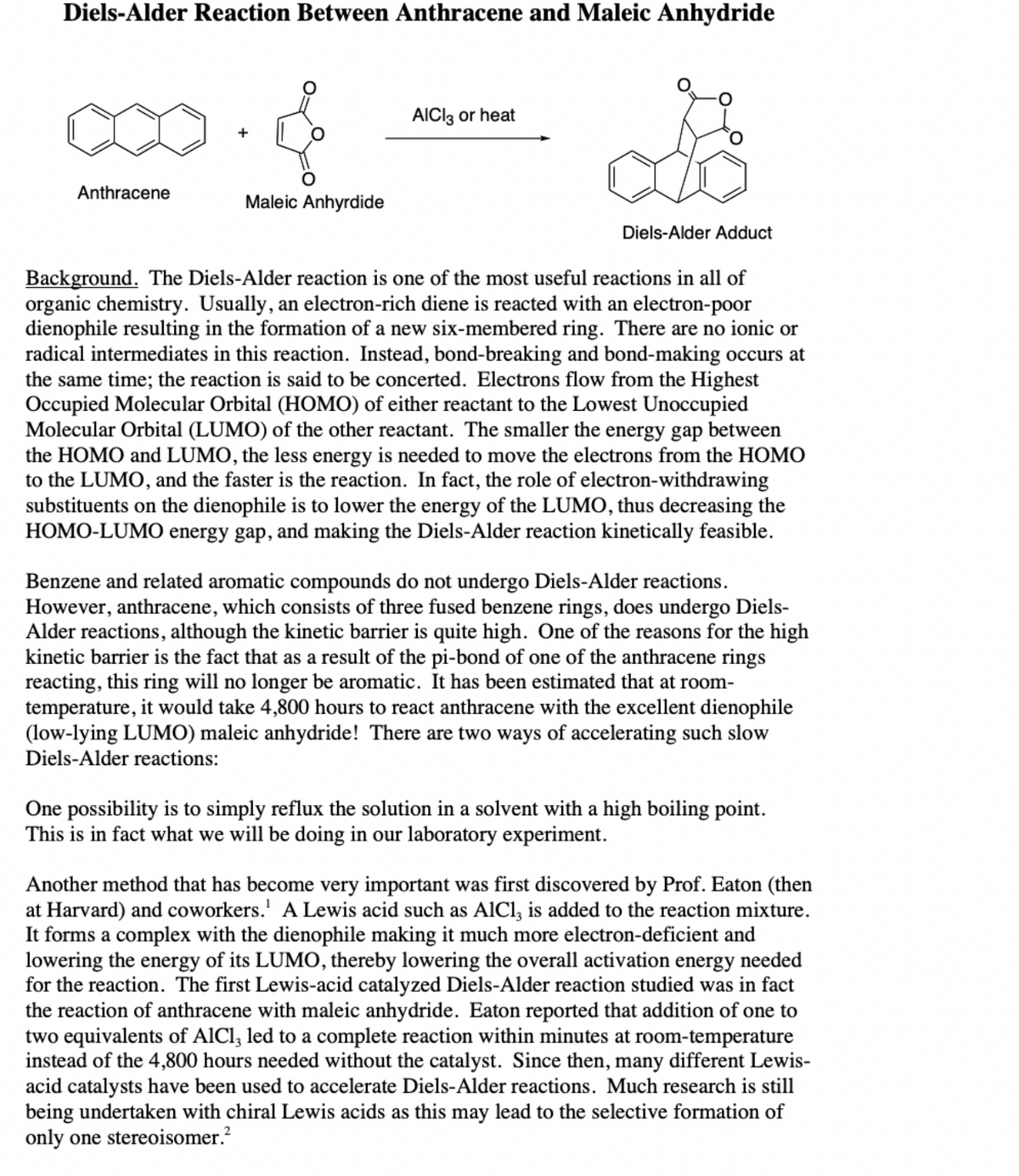
Transcribed Image Text:Diels-Alder Reaction Between Anthracene and Maleic Anhydride
AICI3 or heat
Anthracene
Maleic Anhyrdide
Diels-Alder Adduct
Background. The Diels-Alder reaction is one of the most useful reactions in all of
organic chemistry. Usually, an electron-rich diene is reacted with an electron-poor
dienophile resulting in the formation of a new six-membered ring. There are no ionic or
radical intermediates in this reaction. Instead, bond-breaking and bond-making occurs at
the same time; the reaction is said to be concerted. Electrons flow from the Highest
Occupied Molecular Orbital (HOMO) of either reactant to the Lowest Unoccupied
Molecular Orbital (LUMO) of the other reactant. The smaller the energy gap between
the HOMO and LUMO, the less energy is needed to move the electrons from the HOMO
to the LUMO, and the faster is the reaction. In fact, the role of electron-withdrawing
substituents on the dienophile is to lower the energy of the LUMO, thus decreasing the
HOMO-LUMO energy gap, and making the Diels-Alder reaction kinetically feasible.
Benzene and related aromatic compounds do not undergo Diels-Alder reactions.
However, anthracene, which consists of three fused benzene rings, does undergo Diels-
Alder reactions, although the kinetic barrier is quite high. One of the reasons for the high
kinetic barrier is the fact that as a result of the pi-bond of one of the anthracene rings
reacting, this ring will no longer be aromatic. It has been estimated that at room-
temperature, it would take 4,800 hours to react anthracene with the excellent dienophile
(low-lying LUMO) maleic anhydride! There are two ways of accelerating such slow
Diels-Alder reactions:
One possibility is to simply reflux the solution in a solvent with a high boiling point.
This is in fact what we will be doing in our laboratory experiment.
Another method that has become very important was first discovered by Prof. Eaton (then
at Harvard) and coworkers.' A Lewis acid such as AlCl, is added to the reaction mixture.
It forms a complex with the dienophile making it much more electron-deficient and
lowering the energy of its LUMO, thereby lowering the overall activation energy needed
for the reaction. The first Lewis-acid catalyzed Diels-Alder reaction studied was in fact
the reaction of anthracene with maleic anhydride. Eaton reported that addition of one to
two equivalents of AlCl, led to a complete reaction within minutes at room-temperature
instead of the 4,800 hours needed without the catalyst. Since then, many different Lewis-
acid catalysts have been used to accelerate Diels-Alder reactions. Much research is still
being undertaken with chiral Lewis acids as this may lead to the selective formation of
only one stereoisomer.?
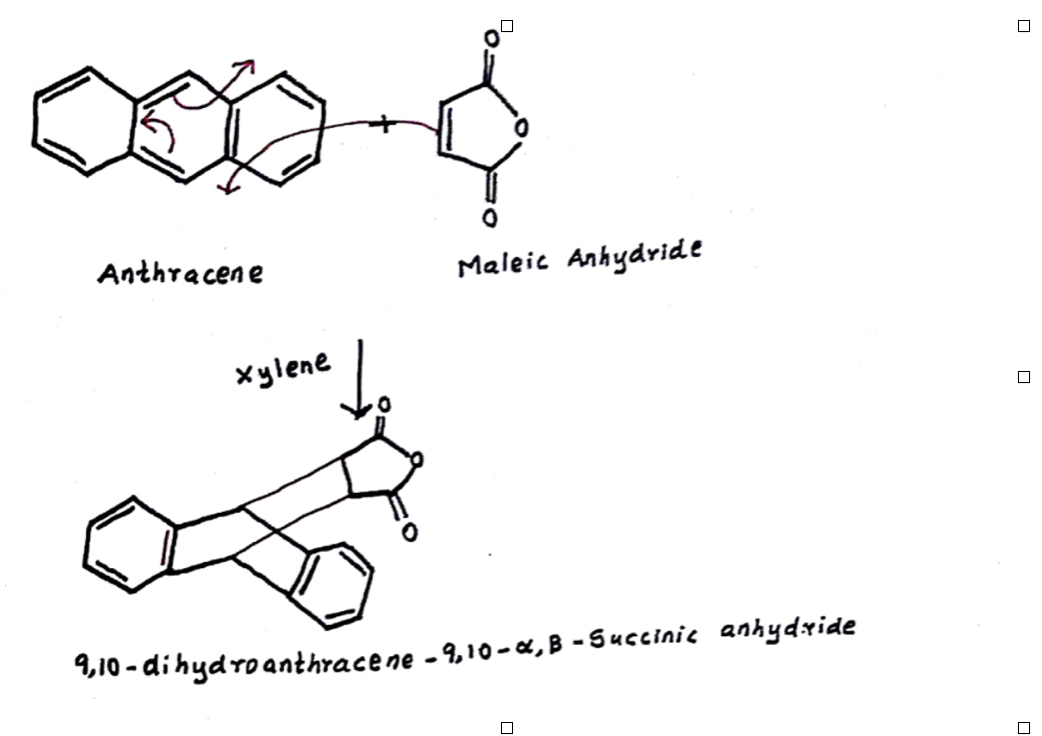
Transcribed Image Text:Anthracene
Maleic Anhydride
Xylene
4,10 - di hydro anthracene - 9,10 – «,B - 5uccinic anhydxide
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning