latasha was trying to identify 452.1 g of an unknown lectrolyte sample. Given the limited instruments in their laborat he decided to mix the sample into 500.0 mL of water to detern e sample's effect on its boiling point and freezing point. The t hows the data she collected for the experiment (Kb= 0.512 ° nd Kf= 1.86 Trial 1 (°C) Trial 2 (°C) Trial 3 (°C) Mean (°C) Boiling point 104.20 103.80 104.10 104.03 Freezing point -14.71 -14.65 -14.63 -14.66 What is the molality of the unknown sample? ) 8.9 m 9.1 m 10.5 m
latasha was trying to identify 452.1 g of an unknown lectrolyte sample. Given the limited instruments in their laborat he decided to mix the sample into 500.0 mL of water to detern e sample's effect on its boiling point and freezing point. The t hows the data she collected for the experiment (Kb= 0.512 ° nd Kf= 1.86 Trial 1 (°C) Trial 2 (°C) Trial 3 (°C) Mean (°C) Boiling point 104.20 103.80 104.10 104.03 Freezing point -14.71 -14.65 -14.63 -14.66 What is the molality of the unknown sample? ) 8.9 m 9.1 m 10.5 m
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 47P
Related questions
Question
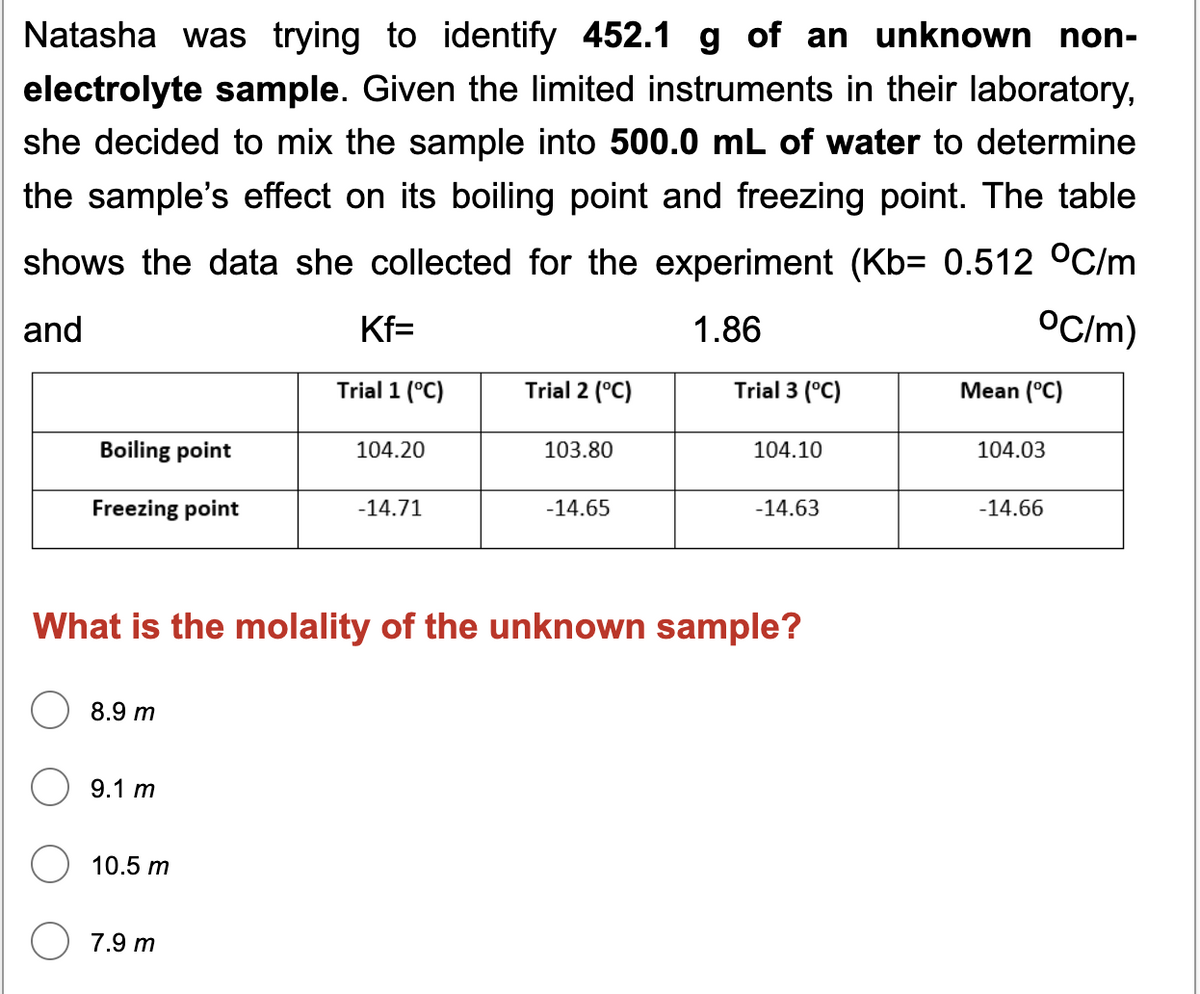
Transcribed Image Text:Natasha was trying to identify 452.1 g of an unknown non-
electrolyte sample. Given the limited instruments in their laboratory,
she decided to mix the sample into 500.0 mL of water to determine
the sample's effect on its boiling point and freezing point. The table
shows the data she collected for the experiment (Kb= 0.512 °C/m
and
Kf=
1.86
OC/m)
Trial 1 (°C)
Trial 2 (°C)
Trial 3 (°C)
Mean (°C)
Boiling point
104.20
103.80
104.10
104.03
Freezing point
-14.71
-14.65
-14.63
-14.66
What is the molality of the unknown sample?
8.9 m
9.1 m
10.5 m
7.9 m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co