Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter5: Resonance
Section: Chapter Questions
Problem 18E
Related questions
Question
I need help with these questions
(Not honor class)
(Not grading)

Transcribed Image Text:2 3.
4 5 6
Types of Chemical Reactions
Do atoms rearrange in predictable patterns during chemical reactions?
Why?
Recognizing patterns allows us to predict future behavior. Weather experts use patterns to
predict dangerous storms so people can get their families to safety. Political analysts use
patterns to predict election outcomes. Similarly, chemists classify chemical equations according
to their patterns to help predict products of unknown but similar chemical reactions.
Model 1 - Types of Reactions
Set A
Set B
4Fe(s) + 30,(g) → 2FE̟O,(s)
N,g) + 3H,(g) → 2NH,(g)
2SO,) + O,g) → 2SO,(g)
MgO(s)+ H¸O(1) → Mg(OH),(aq)
P,O,(g) + 3H,O(1) → 2H,PO,(aq)
So,(g) + H,O(1) → H,SO,(aq)
MgCO,(s) → MgO(s) + CO,(g)
8Li,S(s) → 16L¡(s) + S,(s)
2H,O(1) → 2H,(g) + O,()
2KCIO,(6) → 2KCI(s) + 30,(g)
2Na¸O,(s) → 2Na,0(s) + O,(g)
(NH),CO,(6) → 2NH,(g) + H,O() + CO,2)
Set C
Set D
2FECI,(aq) + 3Zn(s) → 2Fe(s) + 3ZnCl,(aq)
2AI(NO,),(aq) + 3Ca(s) → 3Ca(NO,),(aq) + 2Al(s)
Mg(s) + CuSO,(aq) → MgSO,(aq) + Cu(s)
2AI(s) + 6HCI(aq) → 2AICI,(aq) + 3H,g)
C@) + 2N&B1(aq) → 2NACI(aq) + Br,(1)
ZnBr,(aq) + F,(g) –→ ZnF,(aq) + Br,(1)
AGNO,(aq) + NaCI(aq) → AgCl(s) + NaNO,(aq)
2HNO,(aq) + Mg(OH),(aq) →
Mg(NO,),(aq) + 2H)
Na,CO,(aq) + CACL (aq) →
CACO,(s) + 2N2CI(aq)
FeS(s) + 2HCI(aq) –→H,S(g) + FeCl,(aq)
HCI(aq) + NaOH(aq) -→ H,O(1) + NaC(aq)
FeBr,(aq) + K,PO,(aq) -→ FEPO,(s) + 3KB1(aq)
80
D00
F3
F4
DII
DD
F5
F6
F7
F8
F9
&
*
4
5
7
9
< cO
Transcribed Image Text:Arial
11
BIUA
2
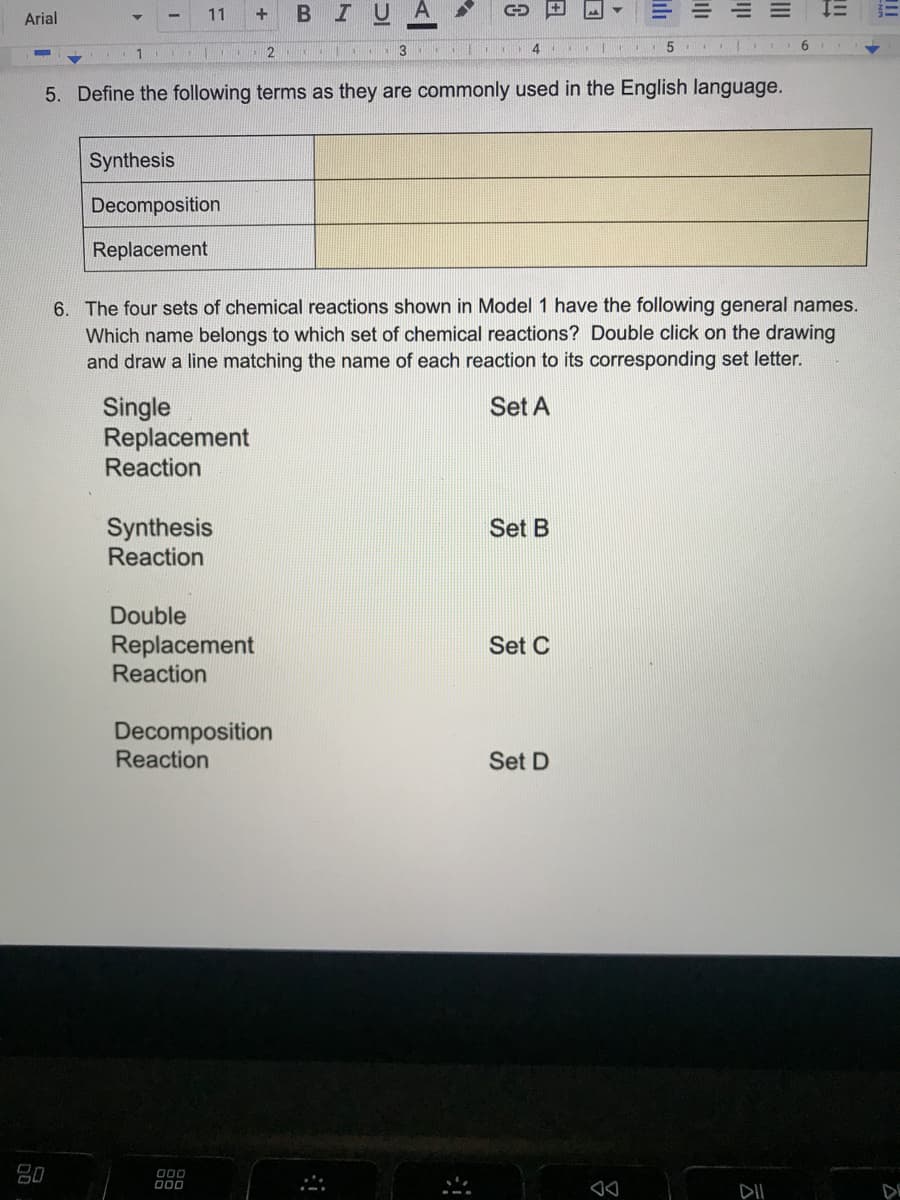
5. Define the following terms as they are commonly used in the English language.
Synthesis
Decomposition
Replacement
6. The four sets of chemical reactions shown in Model 1 have the following general names.
Which name belongs to which set of chemical reactions? Double click on the drawing
and draw a line matching the name of each reaction to its corresponding set letter.
Set A
Single
Replacement
Reaction
Synthesis
Reaction
Set B
Double
Replacement
Reaction
Set C
Decomposition
Reaction
Set D
000
D00
DII
DE
II
日
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax
