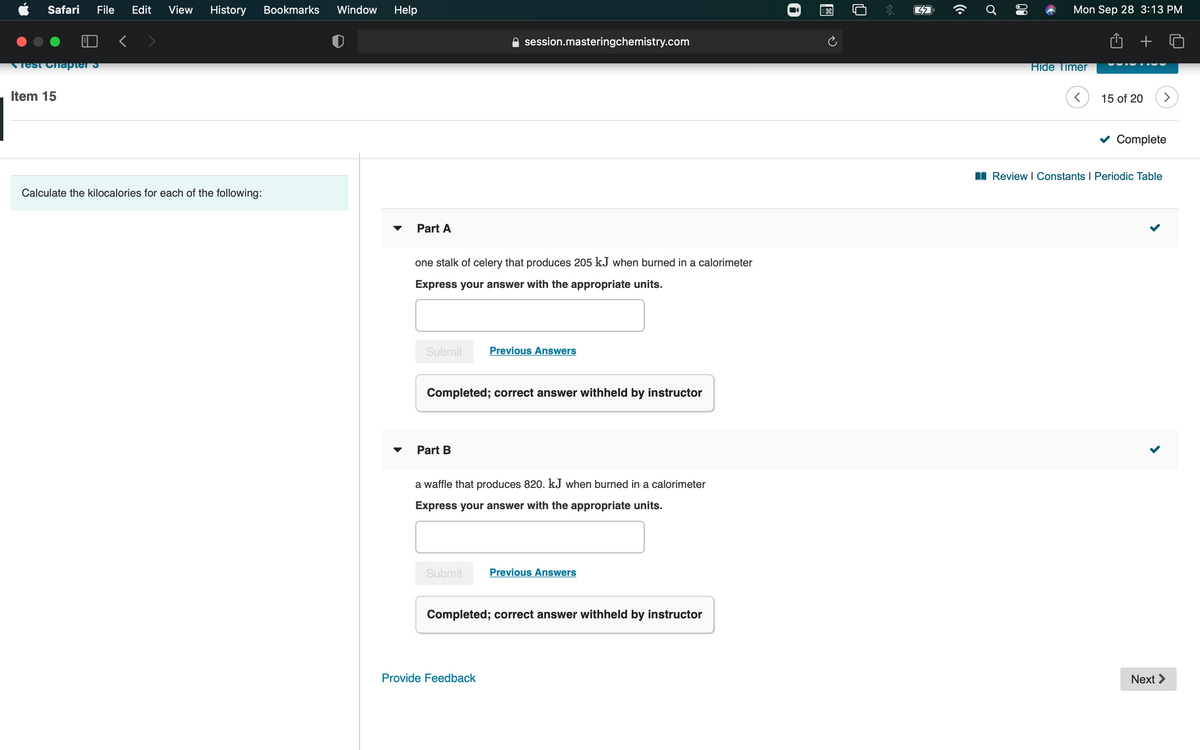
I Review I Constants I Periodic Table Calculate the kilocalories for each of the following: Part A one stalk of celery that produces 205 kJ when burned in a calorimeter Express your answer with the appropriate units. Submit Previous Answers Completed; correct answer withheld by instructor Part B a waffle that produces 820. kJ when burned in a calorimeter Express your answer with the appropriate units. Submit Previous Answers
I Review I Constants I Periodic Table Calculate the kilocalories for each of the following: Part A one stalk of celery that produces 205 kJ when burned in a calorimeter Express your answer with the appropriate units. Submit Previous Answers Completed; correct answer withheld by instructor Part B a waffle that produces 820. kJ when burned in a calorimeter Express your answer with the appropriate units. Submit Previous Answers
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.79PAE: A student performing a calorimetry experiment combined 100.0 ml. of 0.50 M HCI and 100.0 ml. of 0.50...
Related questions
Question
100%
Transcribed Image Text:Safari
File
Edit
View
History
Bookmarks
Window
Help
Mon Sep 28 3:13 PM
session.masteringchemistry.com
Test Chapter 5
Hide Timer
Item 15
15 of 20
v Complete
I Review I Constants I Periodic Table
Calculate the kilocalories for each of the following:
Part A
one stalk of celery that produces 205 kJ when burned in a calorimeter
Express your answer with the appropriate units.
Submit
Previous Answers
Completed; correct answer withheld by instructor
Part B
a waffle that produces 820. kJ when burned in a calorimeter
Express your answer with the appropriate units.
Submit
Previous Answers
Completed; correct answer withheld by instructor
Provide Feedback
Next >
00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning