I Review I Constants I Periodic Table Write the correct ionic formula for the compound formed between the following: Part D Sc+ and O?- Express your answer as a chemical formula. μ ΑΣφ
I Review I Constants I Periodic Table Write the correct ionic formula for the compound formed between the following: Part D Sc+ and O?- Express your answer as a chemical formula. μ ΑΣφ
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter2: Chemical Compounds
Section: Chapter Questions
Problem 60QRT
Related questions
Question
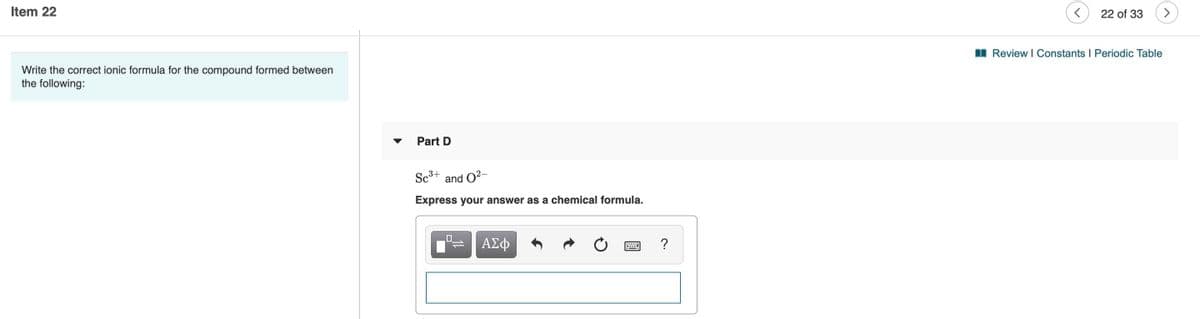
Transcribed Image Text:Item 22
22 of 33
Write the correct ionic formula for the compound formed between
the following:
I Review I Constants I Periodic Table
Part D
Sc3+
and O2-
Express your answer as a chemical formula.
ΑΣφ
?
Expert Solution
Explanation:
Given ions are
Sc+3 and O-2
According to cris- cross method
step1: Cation Anion
Sc O
Step2: charges on the ions
+3 -2
Step3:interchange the charges to opposite side right side bottom
Sc2 O3
Formula of the compound Sc2O3
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
