Chapter20: Applications Of Oxidation/reduction Titrations
Section: Chapter Questions
Problem 20.4QAP
Related questions
Question
I would need help finding the average molarity
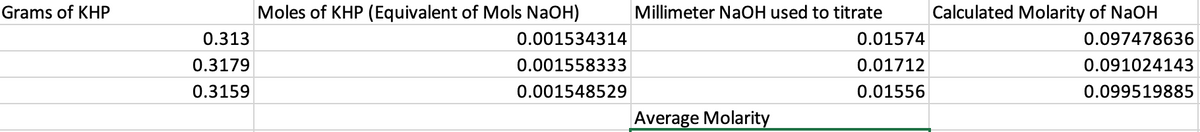
Transcribed Image Text:Grams of KHP
0.313
0.3179
0.3159
Moles of KHP (Equivalent of Mols NaOH)
0.001534314
0.001558333
0.001548529
Millimeter NaOH used to titrate
Average Molarity
0.01574
0.01712
0.01556
Calculated Molarity of NaOH
0.097478636
0.091024143
0.099519885
Expert Solution
Step 1
The average of a set of numbers is simply the sum of the numbers divided by the total number of values in the set.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning