i. Draw Lewis structures for the following and determine what kind of hybrid orbitals the central atom uses in bond formation: (a) ; (b) SO;; and (c) OF,.
i. Draw Lewis structures for the following and determine what kind of hybrid orbitals the central atom uses in bond formation: (a) ; (b) SO;; and (c) OF,.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter10: Molecular Geometry And Chemical Bonding Theory
Section10.6: Electron Configurations Of Diatomic Moleucles Of The Second-period Elements
Problem 10.8E: The C2 molecule exists in the vapor phase over carbon at high temperature. Describe the molecular...
Related questions
Question
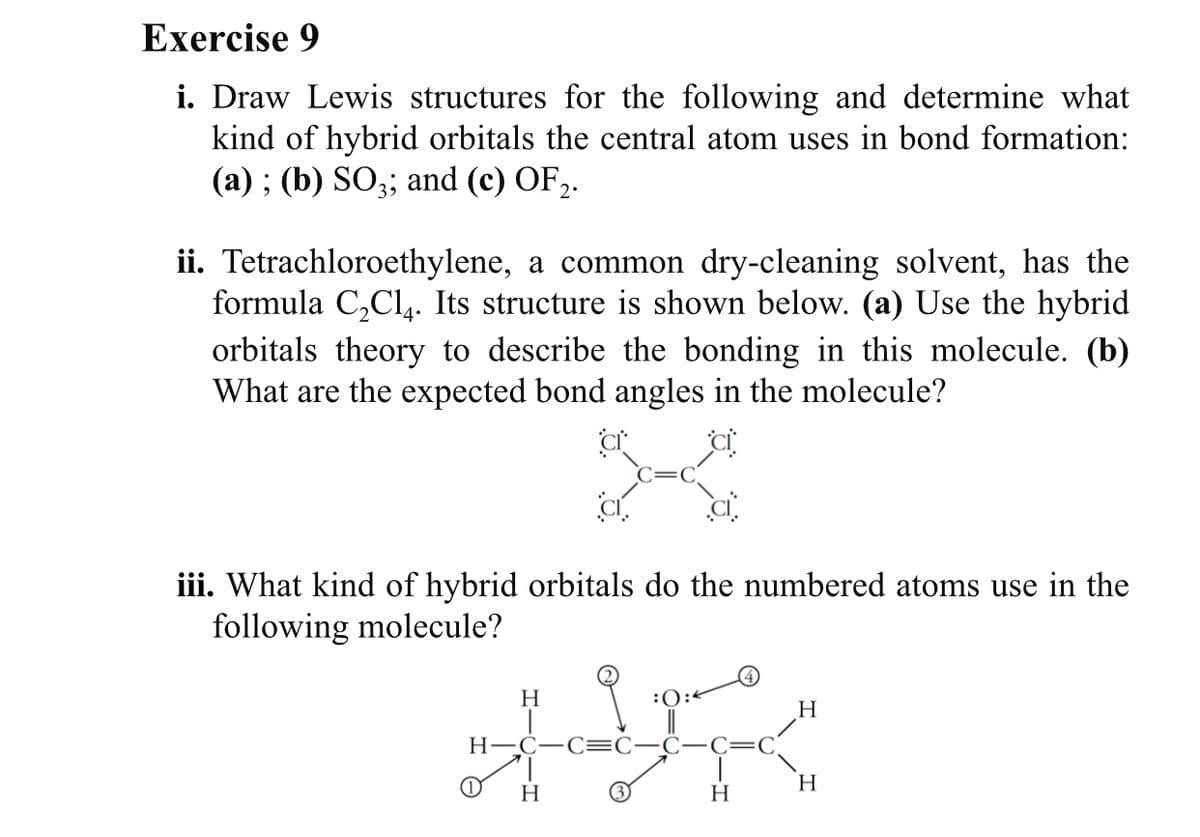
Transcribed Image Text:Exercise 9
i. Draw Lewis structures for the following and determine what
kind of hybrid orbitals the central atom uses in bond formation:
(а) ; (b) SO;3; and (c) OF>.
ii. Tetrachloroethylene, a common dry-cleaning solvent, has the
formula C,Cl,. Its structure is shown below. (a) Use the hybrid
orbitals theory to describe the bonding in this molecule. (b)
What are the expected bond angles in the molecule?
iii. What kind of hybrid orbitals do the numbered atoms use in the
following molecule?
H
:0:<
Н—С—С:
(1)
H
H
H
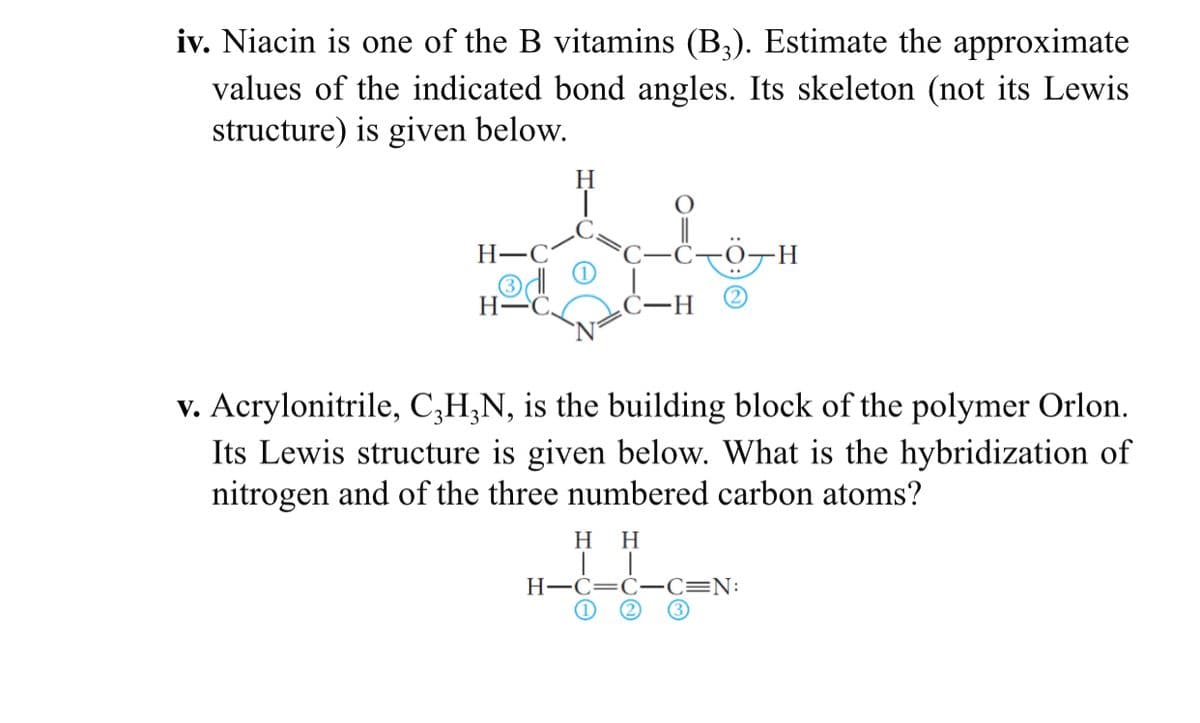
Transcribed Image Text:iv. Niacin is one of the B vitamins (B3). Estimate the approximate
values of the indicated bond angles. Its skeleton (not its Lewis
structure) is given below.
H
Н—С
H.
H-
-H-
v. Acrylonitrile, C;H;N, is the building block of the polymer Orlon.
Its Lewis structure is given below. What is the hybridization of
nitrogen and of the three numbered carbon atoms?
нн
H-C=C- C=N:
(1
(2)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry In Focus
Chemistry
ISBN:
9781305084476
Author:
Tro, Nivaldo J., Neu, Don.
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry In Focus
Chemistry
ISBN:
9781305084476
Author:
Tro, Nivaldo J., Neu, Don.
Publisher:
Cengage Learning