(i.e., A or B) is most soluble in water? Briefly explain your choice. 5- Which compound in each pair .O он HO OH HO O. : O но OH но он Answer Answer: )- Draw the structures for the least and most polar molecules (i.e., isomers) of Bromo-chloro-flouro- iodoethene (C2BrCIFI). Briefly explain your choice. Structure of least polar: Structure of most polar:
(i.e., A or B) is most soluble in water? Briefly explain your choice. 5- Which compound in each pair .O он HO OH HO O. : O но OH но он Answer Answer: )- Draw the structures for the least and most polar molecules (i.e., isomers) of Bromo-chloro-flouro- iodoethene (C2BrCIFI). Briefly explain your choice. Structure of least polar: Structure of most polar:
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter21: Organic Chemistry
Section: Chapter Questions
Problem 99E
Related questions
Question
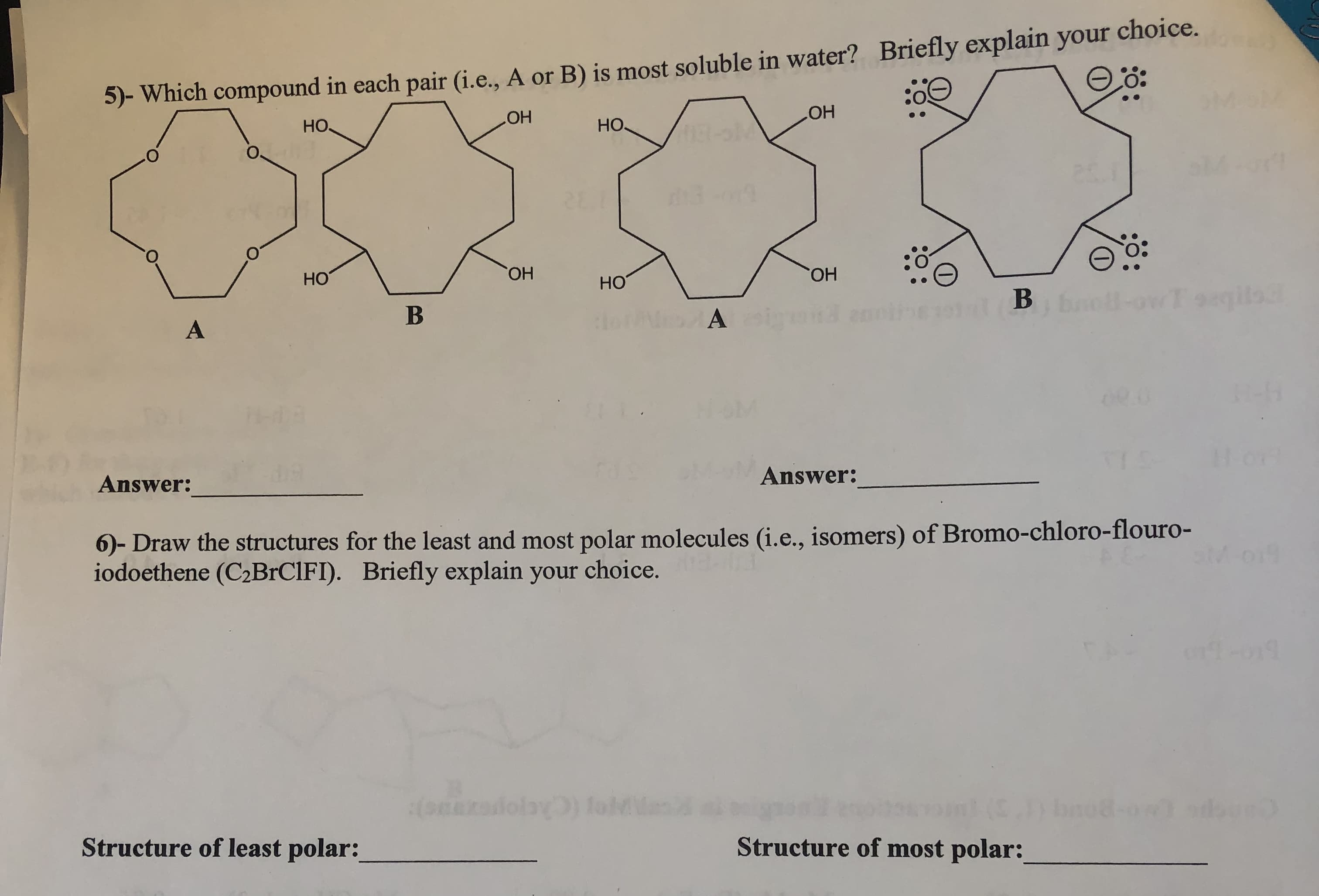
Transcribed Image Text:(i.e., A or B) is most soluble in water? Briefly explain your choice.
5- Which compound in each pair
.O
он
HO
OH
HO
O.
: O
но
OH
но
он
Answer
Answer:
)- Draw the structures for the least and most polar molecules (i.e., isomers) of Bromo-chloro-flouro-
iodoethene (C2BrCIFI). Briefly explain your choice.
Structure of least polar:
Structure of most polar:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning