ich substance would have a higher boiling point: Su termolecular forces that would be present for Substa htermolecular forces that would be present for Substa ing your evidence above, further explain your reasonin g substances would have a higher boiling point. HIS BELOW IN THE BOX PROVIDED TO HELP GUIDE 151
ich substance would have a higher boiling point: Su termolecular forces that would be present for Substa htermolecular forces that would be present for Substa ing your evidence above, further explain your reasonin g substances would have a higher boiling point. HIS BELOW IN THE BOX PROVIDED TO HELP GUIDE 151
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter9: Liquids And Solids
Section: Chapter Questions
Problem 121CWP: Which of the following compound(s) exhibit only London dispersion intermolecular forces? Which...
Related questions
Question
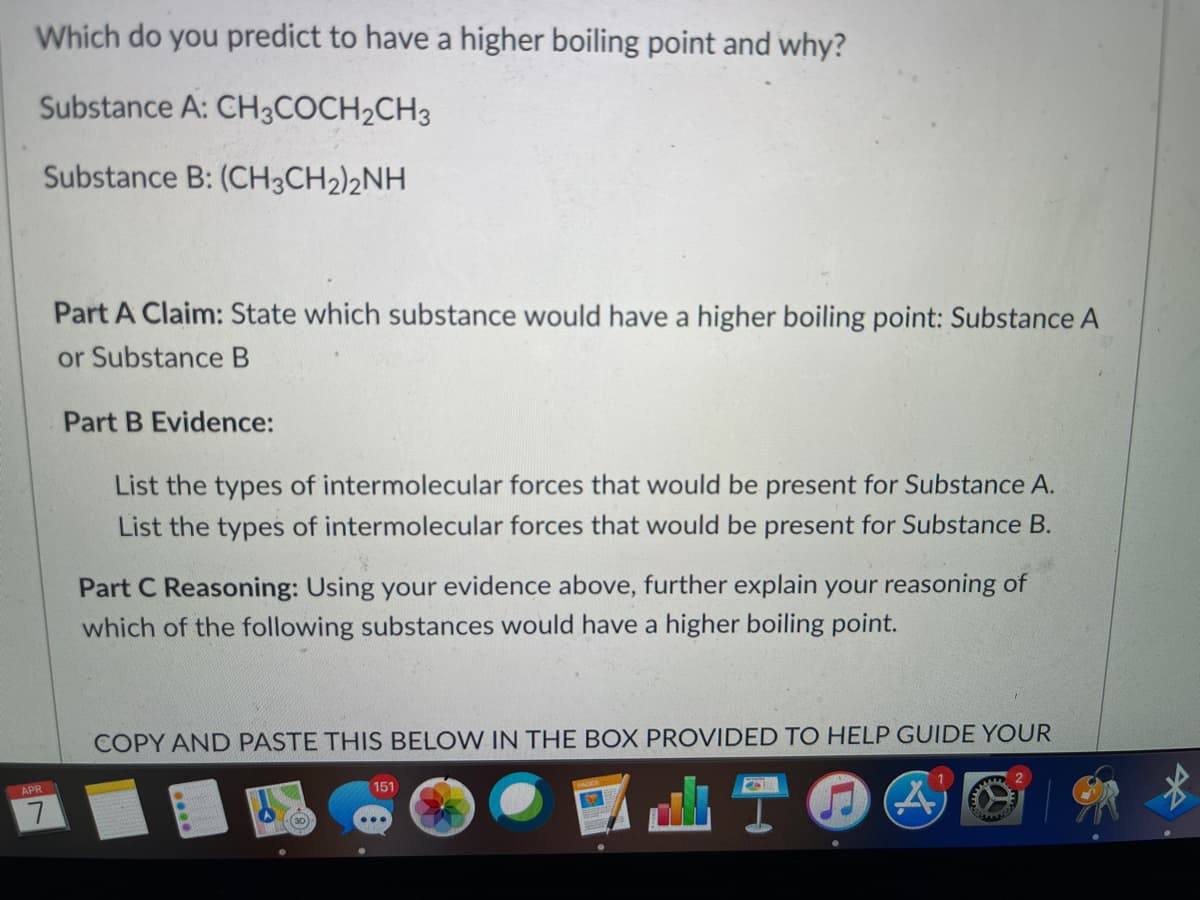
Transcribed Image Text:Which do you predict to have a higher boiling point and why?
Substance A: CH3COCH2CH3
Substance B: (CH3CH2)2NH
Part A Claim: State which substance would have a higher boiling point: Substance A
or Substance B
Part B Evidence:
List the types of intermolecular forces that would be present for Substance A.
List the types of intermolecular forces that would be present for Substance B.
Part C Reasoning: Using your evidence above, further explain your reasoning of
which of the following substances would have a higher boiling point.
COPY AND PASTE THIS BELOW IN THE BOX PROVIDED TO HELP GUIDE YOUR
APR
151
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning