Identify each of the following half-reactions as either an oxidation half-reaction or a reduction half-reaction. Write a balanced equation for the overall redox reaction. Use smallest possible integer co
Identify each of the following half-reactions as either an oxidation half-reaction or a reduction half-reaction. Write a balanced equation for the overall redox reaction. Use smallest possible integer co
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter19: Oxidation-reduction(electron Transfer) Reactions
Section: Chapter Questions
Problem 2PE
Related questions
Question
Identify each of the following half-reactions as either an oxidation half-reaction or a reduction half-reaction.
Write a balanced equation for the overall
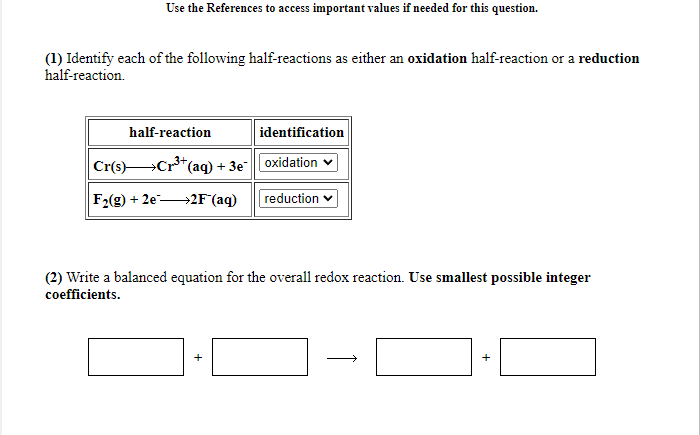
Transcribed Image Text:Use the References to access important values if needed for this question.
(1) Identify each of the following half-reactions as either an oxidation half-reaction or a reduction
half-reaction.
half-reaction
identification
Cr(s)Cr*(
"(aq) + 3e"oxidation v
F2(g) + 2e-
→2F°(aq)
reduction
(2) Write a balanced equation for the overall redox reaction. Use smallest possible integer
coefficients.
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning