Identify the INCORRECT Statement below: A The electron and the proton have charges equal in magnitude and opposite in sign. B Equal masses of different elements contain equal numbers of atoms. The atomic number is the number of protons in the nucleus. The number above the element symbol on the periodic chart is the atomic number. Avogadro's number is the number of atoms in exactly 12 grams of Carbon- 12.
Identify the INCORRECT Statement below: A The electron and the proton have charges equal in magnitude and opposite in sign. B Equal masses of different elements contain equal numbers of atoms. The atomic number is the number of protons in the nucleus. The number above the element symbol on the periodic chart is the atomic number. Avogadro's number is the number of atoms in exactly 12 grams of Carbon- 12.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 77QAP
Related questions
Question
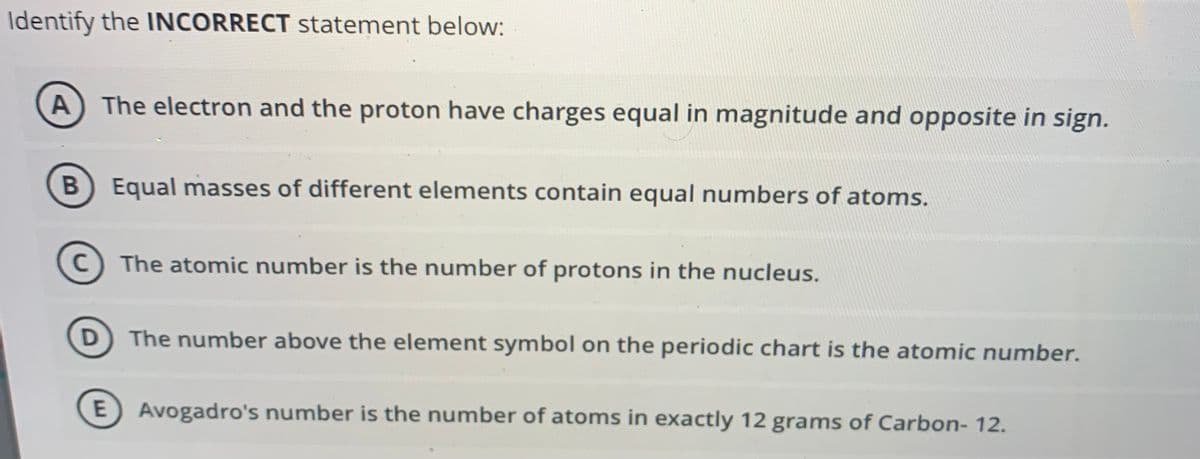
Transcribed Image Text:Identify the INCORRECT statement below:
A) The electron and the proton have charges equal in magnitude and opposite in sign.
B Equal masses of different elements contain equal numbers of atoms.
C) The atomic number is the number of protons in the nucleus.
D The number above the element symbol on the periodic chart is the atomic number.
E) Avogadro's number is the number of atoms in exactly 12 grams of Carbon- 12.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning