Identify the last occupied sublevel, which can be completely or partially filled, for each of the following elements. Drag the appropriate items to their appropriate bins. View Available Hint(s) Reset Help MgPm Zn Sn Am w K CI 3s Зр 3d 4s 4f 5p 5d 5f
Identify the last occupied sublevel, which can be completely or partially filled, for each of the following elements. Drag the appropriate items to their appropriate bins. View Available Hint(s) Reset Help MgPm Zn Sn Am w K CI 3s Зр 3d 4s 4f 5p 5d 5f
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter11: Atomic Theory :the Quantum Model Of The Atom
Section: Chapter Questions
Problem 52E
Related questions
Question
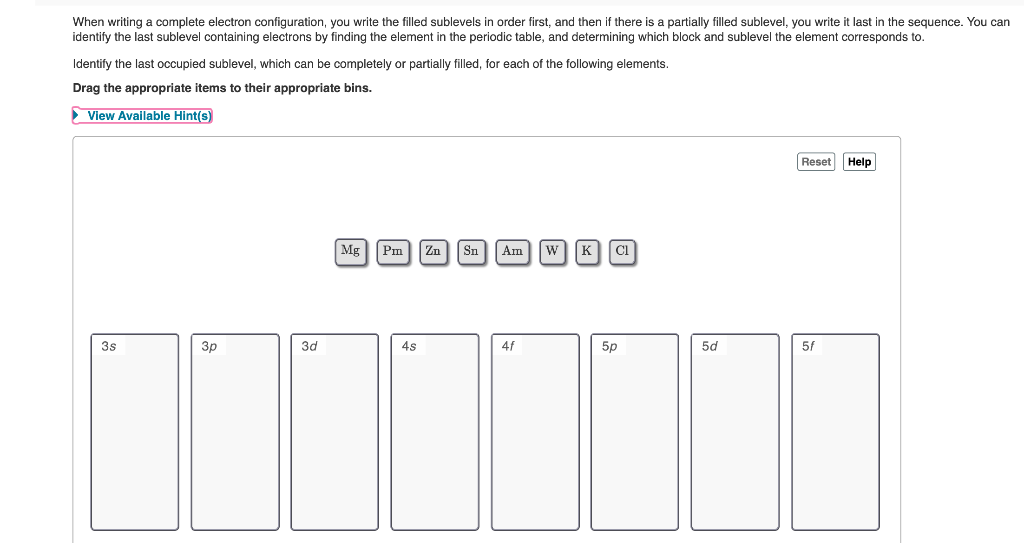
Transcribed Image Text:When writing a complete electron configuration, you write the filled sublevels in order first, and then if there is a partially filled sublevel, you write it last in the sequence. You can
identify the last sublevel containing electrons by finding the element in the periodic table, and determining which block and sublevel the element corresponds to.
Identify the last occupied sublevel, which can be completely or partially filled, for each of the following elements.
Drag the appropriate items to their appropriate bins.
View Available Hint(s)
Reset Help
Mg
Pm
Zn| Sn
Am
w[K CI
3s
3p
3d
4s
4f
5p
5d
5f
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning