Identify the possible their position isomerism. Indicate the formation of compound N from compound K. Predict the chemical reaction that occurs when compound L reacts with 2,4- dinitrophenylhydrazine.
Identify the possible their position isomerism. Indicate the formation of compound N from compound K. Predict the chemical reaction that occurs when compound L reacts with 2,4- dinitrophenylhydrazine.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter22: Reactions Of Benzene And Its Derivatives
Section: Chapter Questions
Problem 22.51P
Related questions
Question
100%
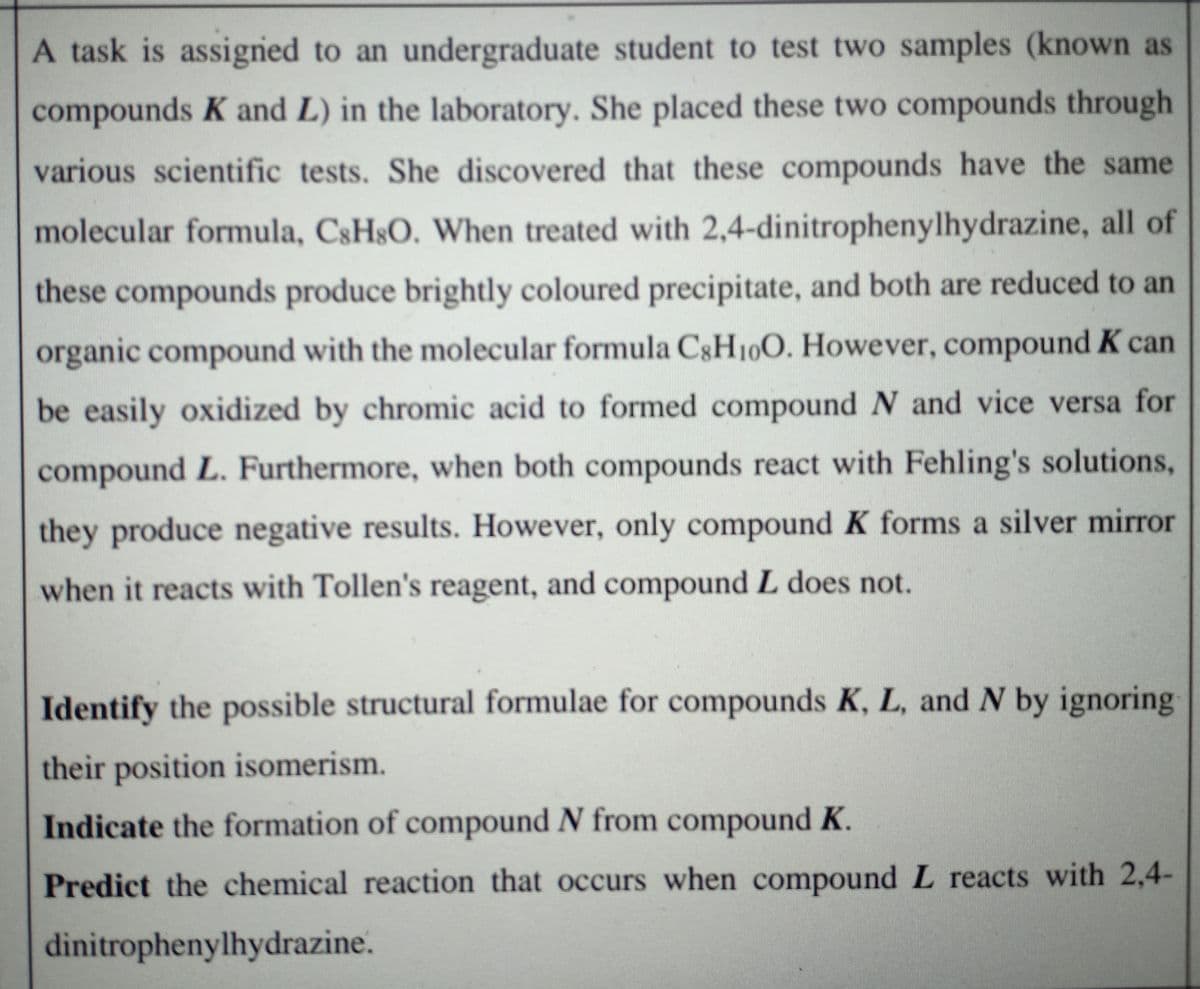
Transcribed Image Text:A task is assigned to an undergraduate student to test two samples (known as
compounds K and L) in the laboratory. She placed these two compounds through
various scientific tests. She discovered that these compounds have the same
molecular formula, CSHSO. When treated with 2,4-dinitrophenylhydrazine, all of
these compounds produce brightly coloured precipitate, and both are reduced to an
organic compound with the molecular formula C§H100. However, compound K can
be easily oxidized by chromic acid to formed compound N and vice versa for
compound L. Furthermore, when both compounds react with Fehling's solutions,
they produce negative results. However, only compound K forms a silver mirror
when it reacts with Tollen's reagent, and compound L does not.
Identify the possible structural formulae for compounds K, L, and N by ignoring
their position isomerism.
Indicate the formation of compound N from compound K.
Predict the chemical reaction that occurs when compound L reacts with 2,4-
dinitrophenylhydrazine.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole