Identify the spectator ions in the following precipitation reaction. Pb(NO3)4(aa) + Na,SO4(ao)> Pb(SO4)215) + NANO3(ag) Group of answer choices Pb4+ and SO42- Nat and NO3 All of them Na* and SO42- Pb4+ and NO3- Determine the difference in the oxidation number of the chromium atom in and chromate CrO42-. Group of answer choices 1 4 CCC C
Identify the spectator ions in the following precipitation reaction. Pb(NO3)4(aa) + Na,SO4(ao)> Pb(SO4)215) + NANO3(ag) Group of answer choices Pb4+ and SO42- Nat and NO3 All of them Na* and SO42- Pb4+ and NO3- Determine the difference in the oxidation number of the chromium atom in and chromate CrO42-. Group of answer choices 1 4 CCC C
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 89SCQ
Related questions
Question
Transcribed Image Text:Document2 - Word
O Search
References
Mailings
Review
View
Help
Grammarly
Acrobat
O Find -
Aav Ao三、三、i、 M处T
AaBbCcDd AaBbCcDd AABBCC AABBCCD
O Replace
Create anc
P v A v
三、 v田、
1 Normal
1 No Spac. Heading 1 Heading 2
A Select v
Adobe
Paragraph
Styles
Editing
Ado
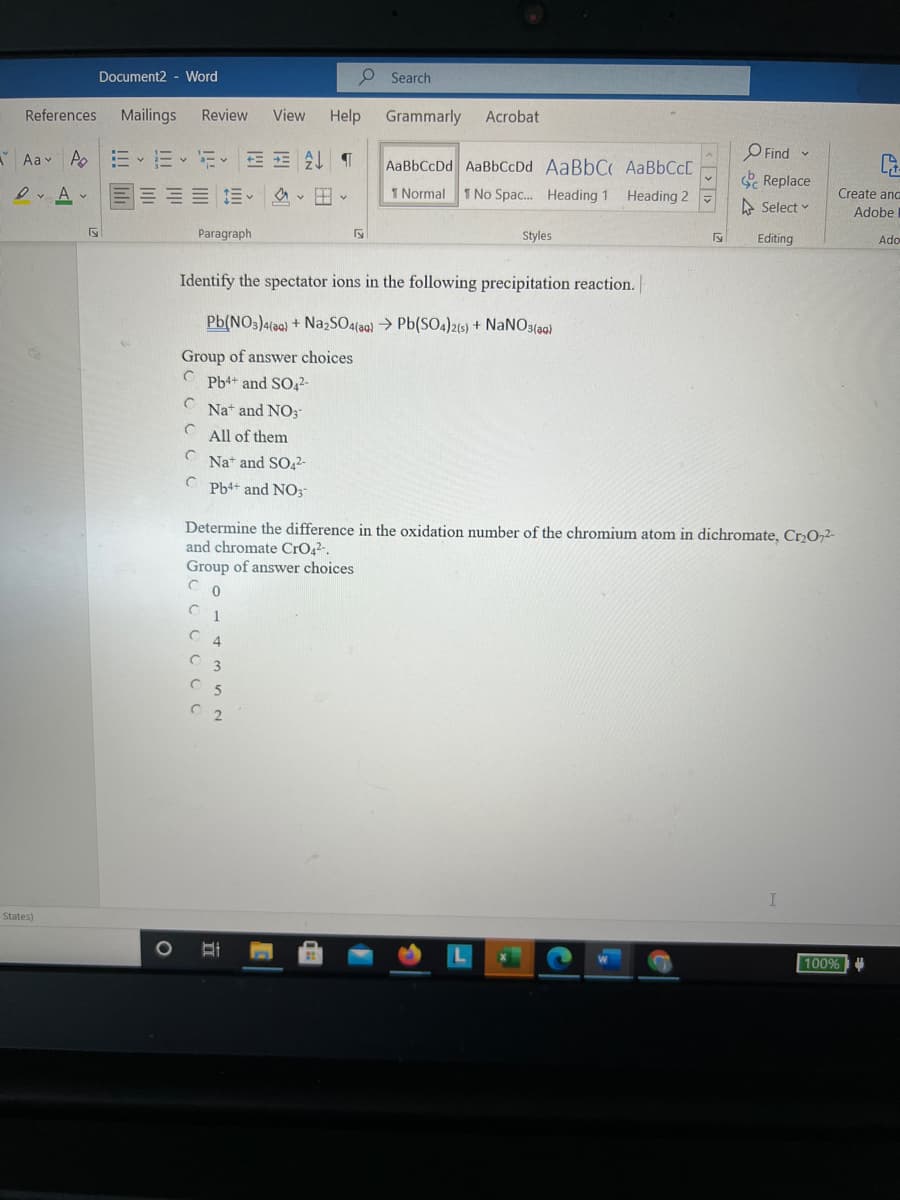
Identify the spectator ions in the following precipitation reaction.
Pb(NO3)4(aa) + Na,SO4(aa) > Pb(SO4)215) + NANO3(a)
Group of answer choices
Pb4+ and SO42-
Nat and NO3-
C All of them
Nat and
Pb4+ and NO3-
Determine the difference in the oxidation number of the chromium atom in dichromate, CrO,2-
and chromate CrO2².
Group of answer choices
4.
C 3
States)
100%
立
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning