Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter21: Benzene And The Concept Of Aromaticity
Section: Chapter Questions
Problem 21.39P
Related questions
Question
Identify the type of bonding that occurs in each solution and determine whether they are conductive or not.
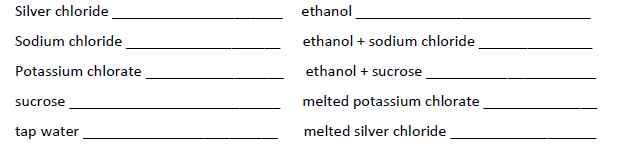
Transcribed Image Text:Silver chloride
Sodium chloride
Potassium chlorate
sucrose
tap water
ethanol
ethanol + sodium chloride
ethanol + sucrose
melted potassium chlorate
melted silver chloride
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Shouldn't the water and sucrose produce dipole-dipole intermolecular forces? Based on our experiment, the solution of water and sucrose conducted electricity as evidenced by the light that was seen on a light bulb. However, it took a few seconds to light and the light was not very bright. What could be the cause of this conduction if covalent bonds cannot conduct electricity?
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning